3048
White matter damage in end-stage kidney disease patients with cognitive impairment: a fixel-based analysis
Chih-Chien Tsai1, YI-Chou Hou2, Yao-Liang Chen3, and Jiun-Jie Wang4
1Healthy Aging Research Center, Chang Gung University, Taoyuan, Taiwan, 2Department of Nephrology, Cardinal Tien Hospital at Xindian, New Taipei City, Taiwan, 3Department of Diagnostic Radiology, Chang Gung Memorial Hospital at Keelung, Keelung, Taiwan, 4Department of Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan, Taiwan
1Healthy Aging Research Center, Chang Gung University, Taoyuan, Taiwan, 2Department of Nephrology, Cardinal Tien Hospital at Xindian, New Taipei City, Taiwan, 3Department of Diagnostic Radiology, Chang Gung Memorial Hospital at Keelung, Keelung, Taiwan, 4Department of Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan, Taiwan
Synopsis
Keywords: Dementia, Kidney
Patients with end-stage kidney disease are vulnerable to cognitive impairment or dementia. Previous studies indicated that end-stage kidney disease subjects manifest prominent white matter hyperintensities. We use fixel-based analysis to examine the tract-specific differences in fixel-based metrics between end-stage kidney disease patients and normal participants. The results demonstrate the pattern of white matter degeneration in end-stage kidney disease patients. Our findings suggest that end-stage kidney disease patients with cognitive impairment exhibit white matter abnormalities. In clinical, the fixel-based analysis may serve as a potential biomarker for monitoring white matter change caused by disease.Introduction
Patients with end-stage kidney disease are vulnerable to cognitive impairment or dementia. Both diseases shared common risk factors, such as insulin resistance or anemia. In addition, Leukoarariosis, known as white matter hyperintensities, is common in end-stage kidney disease subjects 1. The severity of leukoarariosis is associated with cortical cholinergic disaffection and axonal degeneration 2. Such cholinergic dysregulation can further contribute to the cognitive impairment in patients 3, 4. The study aims to investigate if the severity of the comorbidities in end-stage kidney disease is associated with cognitive impairment by axonal degeneration or white matter damage by using diffusion MRI and fixel-based analysis.Methods
The study was approved by the appropriate Institutional Review Board and complied with the Declaration of Helsinki. All participants gave their written informed consent. Total 31 patients with end-stage kidney disease (mean age: 69.7 ± 8.3 years old) were enrolled. Additional 16 normal participants (mean age: 61.1 ± 10.4 years old) were recruited as healthy controls. The Montreal Cognitive assessments was obtained from all participants. A score below 21 was regarded as cognitive impairment. Accordingly, patients were further divided into two groups: cognitive normal (n= 17, mean age: 66.9±7.2 years old) and cognitive impairment (n = 14, mean age: 66.9±7.2 years old).The diffusion-weighted images were collected using a 3T MR scanner (Trio, Siemens, Germany) with a 12-channel head matrix coil with b = 1000 s/mm2 and 64 diffusion-encoding directions. An additional non-diffusion weighted image (b = 0) was acquired.
Fixel-based analysis was performed in MRtrix3 (version 0.3.15) with the recommended procedures 5. The preprocessing included denoising; removal of Gibbs ringing; correction of motion, distortion artifacts; and the bias field. The fiber orientations distribution function (FOD) was subsequently normalized to create a study-specific template. Fixel-specific measures were calculated including Fiber Density (FD) and Fiber Cross-section FC and multiplication of both (FDC).
The statistical analyses of images were performed in MRtrix3.Differences in Fixel-specific measures were evaluated by using connectivity-based fixel enhancement (CFE) and non-parametric permutation testing 6, with age and sex as covariates. The threshold of significance was p < 0.05 after family-wise error correction for multiple comparisons.
Results
Figure 1 showed the difference of FDC in patients when compared to normal participants. Reductions of FDC in patients could be noticed in body and splenium of corpus callosum, corticospinal tract, cerebral peduncle, superior cerebellar peduncle and posterior limb of internal capsule. No significant difference was found between patients with normal cognition and healthy controls.Reduced FDC can be noticed in patients with cognitive impairment when compared to healthy controls, as in Figure 2. The affected regions included body and splenium of corpus callosum, fornix, corticospinal tract, inferior and superior cerebellar peduncle, cerebral peduncle, superior corona radiata, external capsule, and cingulum.
Figure 3 showed the difference in FDC when compared patients with to without cognitive impairment. Reductions of FDC in patients with cognitive impairment can be found in body and splenium of corpus callosum, superior cerebellar peduncle, and cingulum.
Discussion
In patients with end-stage kidney disease, white matter damage was noticed, which might be associated with cognitive impairment. Compromised integrity of the white matter might occur in body and splenium of corpus callosum, as shown by a reduced FDC. This observation, in turn, might be related to comorbidities among these patients. Fixel-based analysis showed that white matter damage can be noticed in patients with end-stage kidney disease, and can be associated with the cognitive impairment.Conclusion
Fixel-based analysis could detect subtle changes in fiber tract within the voxel from end-stage kidney disease patients with cognitive impairment. It might improve our understanding of the damage as occurred in the brain and can be useful in monitoring the possible white matter damage.Acknowledgements
This
research was co-sponsored by Ministry of Science and Technology Taiwan (MOST 109-2221-E-182-009-MY3, MOST
109-2314-B-182-021-MY3); the Healthy Aging Research Center (grant EMRPD1I0501,
EMRPD1I0471, EMRPD1M0451, EMRPD1M0431); and the Chang Gung Memorial Hospital
(CMRPG2J0142, CMRPD1L0141).
References
1. Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). 2007;38:3121-26.2. Bohnen N, Mueller ML, Kuwabara H, Constantine G, Studenski SJN. Age-associated leukoaraiosis and cortical cholinergic deafferentation. 2009;72:1411-16.3. Mazumder MK, Paul R, Bhattacharya P, Borah AJSr. Neurological sequel of chronic kidney disease: From diminished Acetylcholinesterase activity to mitochondrial dysfunctions, oxidative stress and inflammation in mice brain. 2019;9:1-22.4. Hilderman M, Bruchfeld AJNDT. The cholinergic anti-inflammatory pathway in chronic kidney disease—review and vagus nerve stimulation clinical pilot study. 2020;35:1840-52.5. Zhang X, Sun Y, Li W, et al. Characterization of white matter changes along fibers by automated fiber quantification in the early stages of Alzheimer's disease. 2019;22:101723.6. Raffelt DA, Smith RE, Ridgway GR, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. 2015;117:40-55.Figures
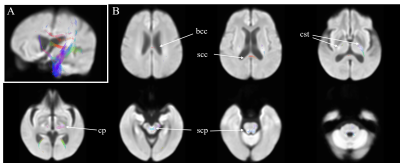
Figure 1. Regions with
significantly reduced fiber density and cross-section in end-stage kidney
disease patients. Panel A: displayed stereoscopically in the superior left
frontal view; Panel B, Axial view. Fixel-based metrics with significance (family-wise error-corrected p <
0.05) were illustrated with color encoded according to the fiber direction: anterior/posterior:
green; superior/inferior: blue; and left/right: red. bcc,
body of corpus callosum; scc: splenium of corpus callosum; cst, corticospinal
tract; cp, cerebral peduncle; scp, superior cerebellar peduncle.
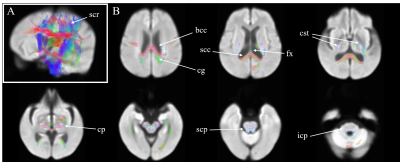
Figure
2. Differences in fiber density and cross-section in end-stage kidney disease
patients with cognitive impairment, when compared to normal participants. Panel A: displayed
stereoscopically in the superior left frontal view; Panel B, Axial view. Color encoded according to the fiber direction: anterior/posterior:
green; superior/inferior: blue; and left/right: red. scr, superior corona radiate; bcc, body of corpus callosum; scc:
splenium of corpus callosum; cg, cingulum; fx, fornix; cst, corticospinal
tract; cp, cerebral peduncle; scp, superior cerebellar peduncle.
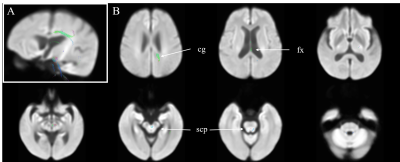
Figure 3. Differences in fixel-based
metrics between end-stage kidney disease patients with or without cognitive
impairment. Panel A: displayed
stereoscopically in the superior left frontal view; Panel B, Axial view. Fixel-based metrics with significance (family-wise error-corrected p <
0.05) were illustrated with color encoded according to the fiber direction: anterior/posterior:
green; superior/inferior: blue; and left/right: red. cg, cingulum; fx,
fornix; scp, superior cerebellar peduncle.
DOI: https://doi.org/10.58530/2023/3048