3047
Predicting brain-behavior relationships using Voxel-based Predictive Modeling in ASL data of older subjects1Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 2Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 3Neurology, University of Michigan, Ann Arbor, MI, United States, 4Psychiatry, University of Michigan, Ann Arbor, MI, United States
Synopsis
Keywords: Alzheimer's Disease, Data Analysis
A recent technique, Connectome-based Predictive Modeling (CPM), has shown promise in relating imaging-derived measures to clinical/behavioral observations. In this work, we adapt this method to relate volumetric CBF data to continuous composite measures of memory, learning, and language in subjects with MCI and DAT, along with healthy subjects. Models using the learning and memory composite scores had the largest effect sizes and statistical significance using permutation testing. Regions in the feature masks indicate involvement of brain regions that may be impacted in DAT. This demonstrates the utility of ASL-based perfusion measurement as a predictor of cognitive status in older subjects.Introduction
Early detection is critical to the intervention of dementia of the Alzheimer’s type (DAT), as DAT-related changes in the brain occur many years before symptoms or diagnosis. One common observation in DAT is the vascular effect of cerebral hypoperfusion1,2. Lower cerebral blood flow (CBF) as measured by ASL has been directly related to cognitive decline and multi-domain impairments in DAT3,4.A recent technique known as Connectome-based Predictive Modeling (CPM) has shown promise in relating fMRI-derived measures to clinical/behavioral observations, and offers a predictive framework, and has shown promise in a DAT population5. Here, we adapt this method to relate volumetric ASL CBF data to continuous composite measures of memory, learning, and language in subjects with MCI and DAT, along with healthy subjects. We hypothesize that the ASL CBF data provides predictive information in relation to these external measures of cognition in DAT.
Methods
Subjects and neuropsychological measures: 90 subjects (Age 79.4±7.2) were enrolled at the Michigan Alzheimer’s Disease Research Center. •Diagnosis was based on consensus conference following criteria used by the National Alzheimer’s Coordinating Center, resulting in diagnoses:- cognitively normal (n=48), amnestic mild cognitive impairment (aMCI; n=22), dementia of the Alzheimer’s type (DAT; n=20). Several composite cognitive measures were used, along with the Montreal Cognitive Assessment (MoCA). Summary of all measures are listed in Table 1.MRI data: Structural T1 MRI and arterial spin labeling-derived cerebral blood flow data were acquired using a Nova Medical 32-channel head coil on a 3T MR750 GE scanner. T1 parameters included an image size of 256x256x208 with 1 mm3 resolution. 3D PCASL parameters included TR/TE of 4.902 s/10.8 ms, post-labelling delay of 2.025 s, matrix of 128x128x40, 1.75mm x1.75mm x3.5mm resolution, 40 slices, 3 NEX.
Preprocessing: ASL data were preprocessed using an automated toolbox, ExploreASL version 1.9.06, including SPM12, CAT127, LST v2.0.158, and with Matlab 2020a. The standard ExploreASL preprocessing pipeline was used, with the option for partial volume correction selected as well. Additionally, normalization to the MNI152 template was performed with SPM12. The resulting CBF maps were used for further analysis. A normalized gray matter tissue segmentation was obtained for each subject using T1w images in SPM12 with the default parameters. To best capture continuous gray matter regions, the gray matter tissue segmentation was smoothed with a Gaussian kernel at 6 mm FWHM and then thresholded at 0.3. Then, the overlapping union of gray matter regions across all subjects was used as the final mask for the entire CBF dataset to ensure the same number of possible features for each subject.
The voxel-wise predictive modeling (VPM) approach is depicted in Figure 1. A CPM-like framework was used; however, rather than connectome edges as input for each subject, VPM uses voxels, specifically voxels from the volumetric ASL CBF maps. In a cross-validation framework, the CBF maps with a gray matter mask were used as voxel-wise predictor input data. Age was regressed from the predictors separately for train and test partitions. First, each voxel (across subjects) in the CBF maps is correlated with response variable (across subjects). Those voxels exceeding the predetermined threshold of significance (p<0.01) are used to generate feature masks. Similar to CPM, this is done separately for positive and negative correlations, resulting in two sets of feature masks (positive and negative). Finally, the input training data values within regions of the feature mask are used to fit a linear model with the training responses, and this model is tested on the held-out set to generate a prediction. After all folds are completed, the model performance is evaluated by correlating predicted and actual responses.
Permutation testing was used to assess significance of the model fit. Labels for the cluster locations identified by VPM were determined using MNI coordinates in WFU PickAtlas9 from the Talairach Daemon and the automated anatomical labeling (AAL) atlas.
Results and Discussion
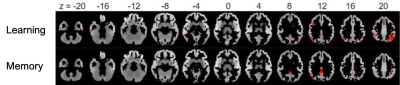
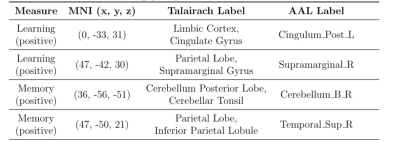
VPM relationships using positive features for all measures are shown in Table 2. Models using the learning and memory composite scores had the largest effect sizes and resulted in statistical significance (p<0.05) using permutation testing. No significant results were found for negative features.Figure 2 shows the corresponding positive feature masks resulting from the models for each of the significant neuropsychological metrics. Regions of the brain isolated in the VPM feature masks (Table 3) indicate involvement of brain regions that may be impacted in DAT. In the learning-related areas, synaptic loss and cortical thinning associated with DAT neuropathology has been shown to occur within the cingulate gyrus10,11; and DAT subjects displayed increased fMRI activations in the supramarginal gyrus compared to controls in working memory tasks12. In the memory-related areas, altered glucose metabolism has been observed in the cerebellar tonsil to indicate a potential for conversion to cognitive decline13, and disruptions in CBF have been specifically observed in the IPL in subjects with DAT14.
Conclusion
This work demonstrates the utility of ASL-based perfusion measurement as a predictor for external measures of cognitive and memory status in patients with varying degrees of DAT.Acknowledgements
This project was supported in part by the National Institute on Aging via Michigan Alzheimer’s Disease Research Center (MADRC; 5P30 AG053760) and R01 AG058724, NIH grant R01 NS 112233, and the University of Michigan Precision Health Investigators Award.References
1. Dede DS, Yavuz B, Yavuz BB, Cankurtaran M, Halil M, Ulger Z, Cankurtaran ES, Aytemir K, Kabakci G, Ariogul S. Assessment of endothelial function in Alzheimer's disease: is Alzheimer's disease a vascular disease?. Journal of the American Geriatrics Society. 55(10):1613-7 (2007).
2. Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, Nakano S, Takasaki M. Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. Journal of nuclear medicine. 41(7):1155-62 (2000).
3. Rodriguez G, Vitali P, Calvini P, Bordoni C, Girtler N, Taddei G, Mariani G, Nobili F. Hippocampal perfusion in mild Alzheimer's disease. Psychiatry Research: Neuroimaging. 100(2):65-74 (2000).
4. Leeuwis AE, Benedictus MR, Kuijer JP, Binnewijzend MA, Hooghiemstra AM, Verfaillie SC, Koene T, Scheltens P, Barkhof F, Prins ND, van der Flier WM. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer's disease. Alzheimer's & Dementia. 13(5):531-40 (2017).
5. Lin Q, Rosenberg MD, Yoo K, Hsu TW, O'Connell TP, Chun MM. Resting-state functional connectivity predicts cognitive impairment related to Alzheimer's disease. Frontiers in aging neuroscience. 10:94 (2018).
6. Mutsaerts HJ, Petr J, Groot P, Vandemaele P, Ingala S, Robertson AD, Václavů L, Groote I, Kuijf H, Zelaya F, O’Daly O. ExploreASL: an image processing pipeline for multi-center ASL perfusion MRI studies. Neuroimage. 219:117031 (2020).
7. Gaser C. Partial volume segmentation with adaptive maximum a posteriori (MAP) approach. NeuroImage. (47):S121 (2009).
8. Schmidt P, Wink L. LST: A lesion segmentation tool for SPM. Manual/Documentation for version. 2:15 (2017).
9. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19(3):1233-9 (2003).
10. Lehmann M, Rohrer JD, Clarkson MJ, Ridgway GR, Scahill RI, Modat M, Warren JD, Ourselin S, Barnes J, Rossor MN, Fox NC. Reduced cortical thickness in the posterior cingulate gyrus is characteristic of both typical and atypical Alzheimer's disease. Journal of Alzheimer's Disease. 2010 Jan 1;20(2):587-98 (2010).
11. Scheff SW, Price DA, Ansari MA, Roberts KN, Schmitt FA, Ikonomovic MD, Mufson EJ. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer's disease. Journal of Alzheimer's Disease. 43(3):1073-90 (2015).
12. Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. European radiology. 16(1):193-206 (2006).
13. Dartora CM, Koole M, da Silva AM. Glucose metabolism changes in cerebellar tonsils as an early predictor of cognitive decline. Alzheimer's & Dementia. 17:e054007 (2021).
14. Verfaillie SC, Adriaanse SM, Binnewijzend MA, Benedictus MR, Ossenkoppele R, Wattjes MP, Pijnenburg YA, van der Flier WM, Lammertsma AA, Kuijer J, Boellaard R. Cerebral perfusion and glucose metabolism in Alzheimer’s disease and frontotemporal dementia: two sides of the same coin?. European radiology. 25(10):3050-9 (2015).
Figures
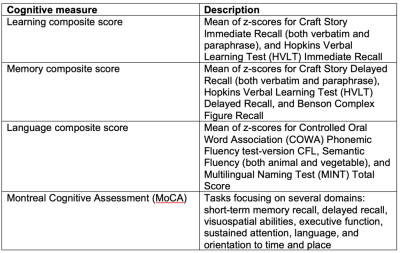
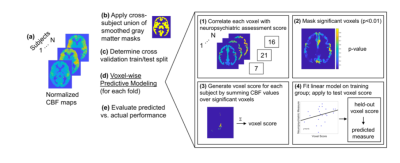
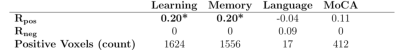
Table 2. ASL VPM results for each neuropsychological measure. Rpos/Rneg is the correlation between predicted and actual measure values for the positive/negative features. Values in bold and starred are significant at p<0.05 using permutation testing.