3044
Quantitative choroid plexus and bulk cerebrospinal fluid metrics in older adults with and without Alzheimer’s disease1Department of Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 3Department of Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Keywords: Alzheimer's Disease, Neurofluids, Aging, Perfusion, Arterial spin labelling
The goal of this work is to characterize changes in the choroid plexus (ChP) and aqueductal cerebrospinal fluid dynamics in patients with Alzheimer’s disease utilizing arterial spin labeling (ASL) and phase contrast (PC), respectively. We observed that across all participants, ChP hypertrophy was associated with reduced ChP perfusion. However, we did not observe differences in ChP perfusion or net CSF flow between AD and healthy cohorts. The observed increase in peak CSF flow with age, despite the general decrease in ChP perfusion with age, suggests that the ChP performs functions beyond simply increasing or decreasing CSF production.
Introduction
The goal of this work is to characterize changes in the choroid plexus (ChP) and aqueductal cerebrospinal fluid dynamics in patients with Alzheimer’s disease (AD) utilizing arterial spin labeling (ASL) and phase contrast (PC), respectively. AD is a progressive, neurodegenerative proteinopathy partially characterized by amyloid beta (Aβ) aggregation. Importantly, cerebrospinal fluid (CSF) production occurs in the ChP complexes, brain waste products are cleared through the CSF circulation, and it has been hypothesized that Aβ retention is partially attributable to CSF circulation dysfunction1,2. Recent work using non-invasive MRI has provide evidence in support of ChP hypertrophy, ChP perfusion reduction, and CSF flow reductions with age across the lifespan (ages=18-86 years)3; the logical extension of this work is to understand whether these changes are disparate in older adults with AD. Here, we apply ASL and PC in AD patients and age-matched controls to test three hypotheses of presumed CSF clearance dysfunction: (1) ChP hypertrophy and perfusion are inversely related, (2) age-related changes in ChP volume and ChP perfusion are accelerated in AD, and (3) CSF net flow decreases in AD.Methods
DemographicsPatients with clinically diagnosed AD and healthy adults provided informed consent and were scanned at 3T using body coil radiofrequency transmission and 32-channel SENSE phased-array reception (Philips) for this prospective study.
Acquisition
The following images were acquired for ChP segmentation: (i) T1-weighted MPRAGE (spatial resolution=1mm3 isotropic) and (ii) T2-weighted FLAIR (spatial resolution=0.6x0.6x5mm3). Perfusion-weighted images were acquired using pseudo-continuous ASL (pCASL): TR/TE=4550/13ms, label duration=1800ms, label delay=2000ms, spatial resolution=3x3x7mm3. ECG-calibrated PC was utilized to quantify aqueductal CSF flow (TR/TE=12/7.8ms; VENC=12cm/s; spatial resolution=0.59x0.59x4mm3).
Analysis and Hypothesis Testing
Hypothesis (1). ChP volume (cm3) was calculated following segmentation generated by a deep learning method trained with T1-weighted and T2-FLAIR-weighted images3. ChP perfusion was calculated from pCASL acquisitions processed according to the ISMRM perfusion study group guidelines4. A generalized linear model (GLM) was utilized to model ChP volume as the dependent variable and ChP perfusion and group as independent variables (Wald-test significance criteria: p<0.05).
Hypothesis (2). Two-tailed Wilcoxon tests were performed to compare ChP volume and perfusion between AD and age-matched healthy participants (significance criteria: p<0.05). GLMs were utilized to model ChP volume and perfusion as separate dependent variables and age, sex, and group as independent variables (Wald-test significance criteria: p<0.05).
Hypothesis (3). CSF flow was calculated from PC3. Peak CSF flow (mL/s) was the maximum flow value reached throughout the cardiac cycle, and net CSF flow (mL/min) was the integral of the of the CSF flow curve multiplied by the participant’s heart rate. Two-tailed Wilcoxon tests were performed to compare peak CSF flow and net CSF flow between AD patients and age-matched controls (significance criteria: p<0.05). GLMs were utilized to model peak CSF flow and net CSF flow as separate dependent variables and age, sex, and group as independent variables (Wald-test significance criteria: p<0.05).
Results
The cohorts enrolled (Table 1) consisted of age-matched healthy (n=32; age=70.0±7.3 years) and AD (n=34; age=70.5±7.4 years) participants.Hypothesis (1). ChP perfusion (p=0.0499) was observed to have a significant relationship with ChP volume (Figure 1) across all participants.
Hypothesis (2). Wilcoxon tests revealed no significant difference in ChP volume (p=0.22) or ChP perfusion (p=0.15) between AD and healthy participants (Figure 2). Regression models showed that ChP volume was significantly elevated in male relative to female participants (p<0.001; Figure 3). Figure 4 displays case examples summarizing the ChP-related trends.
Hypothesis (3). No significant difference in peak CSF flow (p=0.26) or net CSF flow (p=0.46) between AD and healthy participants was observed (Figure 2). Regression models showed that peak CSF flow significantly increased with increasing age (p<0.001; Figure 3).
Discussion
This is one of the first reports of MRI measures of ChP volumetry, ChP perfusion, and aqueductal CSF in older adults with and without AD. We observed that across all participants, ChP hypertrophy was associated with reduced ChP perfusion which is consistent with inverse hemo-metabolic and tissue volume changes across the lifespan in other tissues5. A recent retrospective study found that larger ChP volume was associated with severity of cognitive impairment across the spectrum of AD6. However, contrary to Hypotheses (2-3), we did not observe differences in ChP perfusion or net CSF flow between AD and healthy cohorts. The observed increase in peak CSF flow with age, despite the general decrease in ChP perfusion with age, suggests that the ChP performs functions beyond simply increasing or decrease CSF production. Importantly, preservation of the measured components of bulk CSF flow may also point toward any CSF clearance disorder being more closely associated with perivascular or interstitial flow, relative to changes in net CSF flow through the aqueduct.Conclusion
This study applied ASL, PC, and anatomical MRI to evaluate choroid plexus and aqueductal CSF changes in AD patients and age-matched healthy controls. ChP hypertrophy is associated with reduction in ChP perfusion, and dynamic flow of CSF through the aqueduct is more closely related to age than is ChP activity in this age range. However, results reveal no significant difference in ChP volume, ChP perfusion, peak CSF flow, nor net CSF flow between AD participants and age-matched controls, which supports possible alternative mechanisms for Aβ retention in AD.Acknowledgements
No acknowledgement found.References
1. Boespflug EL, Iliff JJ. The Emerging Relationship Between Interstitial Fluid-Cerebrospinal Fluid Exchange, Amyloid-beta, and Sleep. Biological psychiatry. 2018;83(4):328-36. doi: 10.1016/j.biopsych.2017.11.031.
2. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012; 4:147ra111. doi: 10.1126/scitranslmed.3003748
3. Eisma JJ, McKnight CD, Hett K, Elenberger J, Song AK, Stark AJ, Claassen DO, Donahue MJ. Choroid plexus perfusion and bulk cerebrospinal fluid flow across the adult lifespan. J Cereb Blood Flow Metab. 2022; 271678X221129101. doi: 10.1177/0271678X221129101. Epub ahead of print.
4. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102–116. doi: 10.1002/MRM.25197.
5. Peng SL, Dumas JA, Park DC, Liu P, Filbey FM, McAdams CJ, et al. Age-related increase of resting metabolic rate in the human brain. NeuroImage. 2014;98:176–183. doi: 10.1016/j.neuroimage.2014.04.078
6. Choi JD, Moon Y, Kim HJ, Yim Y, Lee S, & Moon WJ. Choroid Plexus Volume and Permeability at Brain MRI within the Alzheimer Disease Clinical Spectrum. Radiology. 2022; 304(3): 635–645. doi: 10.1148/radiol.212400
Figures

Table 1. Summary statistics for the subjects included in this study. Values are reported as mean ± standard deviation where applicable. (ChP: choroid plexus; CSF: cerebrospinal fluid)
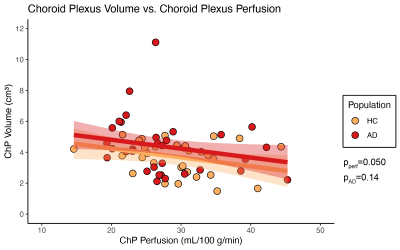
Figure 1. Results from regression analyses with choroid plexus (ChP) volume as the dependent variable in older patients with Alzheimer’s disease (AD) and age-matched healthy controls (HC). ChP perfusion and group were included as covariates. (ChP: choroid plexus; HC: healthy controls; AD: Alzheimer’s disease; pperf: p-value for choroid plexus perfusion beta coefficient; pAD: p-value for group beta coefficient)
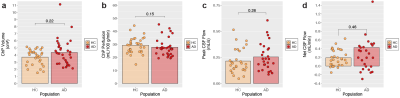
Figure 2. Results from group-wise comparisons of choroid plexus (ChP) volume, ChP perfusion, peak cerebrospinal fluid (CSF) flow, and absolute CSF flow between healthy controls (HC) and patients with Alzheimer’s disease (AD). P-values are shown from a two-tailed Wilcoxon test. (ChP: choroid plexus; CSF: cerebrospinal fluid; HC: healthy controls; AD: Alzheimer’s disease)

Figure 3. Results from regression analyses with choroid plexus (ChP) volume, ChP perfusion, peak cerebrospinal fluid (CSF) flow, and net CSF flow as the dependent variables in older patients with Alzheimer’s disease (AD) and age-matched healthy controls (HC). Age, sex, and group were included as covariates in each model. (ChP: choroid plexus; CSF: cerebrospinal fluid; HC: healthy controls; AD: Alzheimer’s disease; page: p-value for age beta coefficient; psex: p-value for sex beta coefficient; pAD: p-value for group beta coefficient)
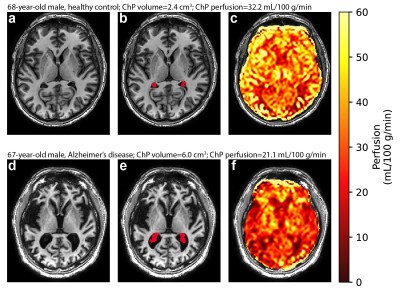
Figure 4. Case examples of choroid plexus volume and perfusion in a 68-year-old, male healthy control (top row: a-c) and a 67-year-old patient with Alzheimer’s disease (bottom row: d-f). In the Alzheimer’s disease patient, an increased choroid plexus volume and decreased choroid plexus perfusion are observed. (ChP: choroid plexus)