3032
Desynchronized white matter function and structure in drug-naive first-episode major depressive disorder patients1Department of Radiology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China, 2Department of Psychiatry, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China, 3Zhejiang Normal University, Jinhua, China
Synopsis
Keywords: White Matter, Psychiatric Disorders
Objective. To describe the abnormalities in WM function and structure in drug-naive first-episode MDD patients.
Methods. 33 patients and 34 HCs were included. Different frequency bands of ALFF and FA were used to analyze the differences between the two groups.
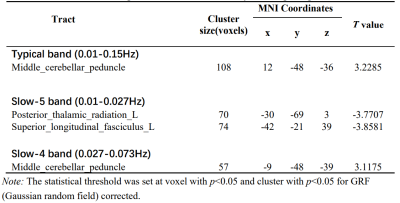
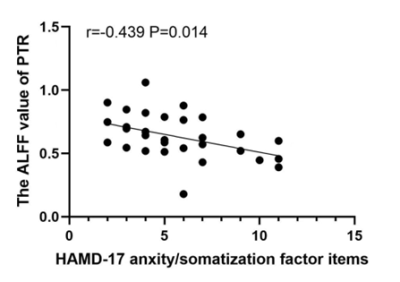
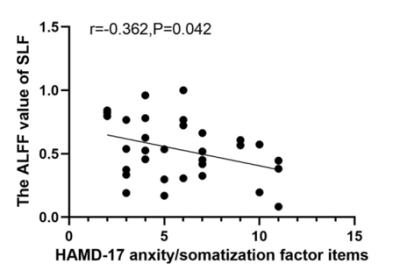
Results. Compared to HCs, patients showed decreased ALFF in PTR and SLF in slow-5 band, which were negatively correlated with the HAMD-17 anxiety/somatization factor items. No difference in FA between two groups.
Conclusions. WM dysfunction relate to the pathophysiological mechanisms of MDD and predates structural damage. Compared to the typical and slow-4 bands, the slow-5 band is more sensitive for early detecting WM dysfunction.
Background
Major depressive disorder (MDD) is a highly prevalent mental disease. Using magnetic resonance imaging (MRI), although numerous studies have revealed the alterations in structure and function of grey matter, few studies focused on the coupling of white matter (WM) structure and function in MDD. The aim of this study was to investigate whether functional and structural abnormalities of WM play an essential role in the neurobiological mechanisms of MDD.Methods
Resting state functional MRI (rsfMRI) data were acquired from 33 drug-naive first-episode MDD patients and 34 healthy controls (HCs). After data preprocessed, amplitude of low frequency fluctuation (ALFF) of WM were calculated, including typical (0.01-0.15Hz), slow-4 (0.027–0.073 Hz) and slow-5 (0.01–0.027 Hz) bands. Fractional anisotropy (FA) was examined in 23 patients and 26 HCs using tract-based spatial statistics (TBSS) based on diffusion tensor imaging. Pearson correlation analysis was applied to analyze the relationships between ALFF values and Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA) scores.Results
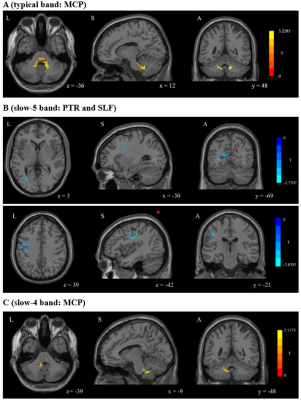
Compared with the HCs, MDD patients showed significantly decreased ALFF values of PTR and SLF in slow-5 band, increased ALFF values of middle cerebellar peduncle (MCP) in typical and slow-4 bands (Table 1 and Figure 1). In TBSS analysis, no significant differences in FA were found between two groups. Further correlation analysis showed that the ALFF values in PTR and SLF were negatively correlated with the HAMD-17 anxiety/somatization factor items (Figure 2, 3).Discussion
PTR is associated with deficits in visual construction, cognitive control, attentional processes, and emotional awareness1.2. Structural abnormalities in PTR have been reported in previous MDD studies3.4.5, but not showed in our study. These inconsistent results may be due to the difference in patient’s condition. The decreased ALFF of PTR suggested reduced spontaneous brain activity in the WM tract connecting the thalamus and occipital cortex, which may be due to impaired transmission in the PTR itself or secondary to structural damage or hypofunction in the thalamus and occipital lobes. Our results favor the latter. Additionally, the negative correlation between the ALFF value of PTR and anxiety/somatization factor items indicated that patients' anxiety and somatic symptoms were worsen as the disease progressed. It has been shown that structural abnormalities in PTR were associated with anxiety symptoms in MDD6 and were present in patients with functional dyspepsia7.The SLF is considered as a higher-order multisensory associative system, which has been reported to be associated with somatic discomfort8, attention9.10 and emotion11. In this study, the reduced ALFF region in SLF was located near the pre/postcentral gyrus at SLF-I, but the abnormality in structure wasn’t found. Previous study has concluded that lower FA values in the SLF are related with more hospitalizations and that SLF fiber integrity may reflect the cumulative disease burden in MDD12. Our results also suggested that the damage in WM structures didn’t occur in the early stage of the disease, but emerged as the disease progresses further. Therefore, we speculate that decreased ALFF values in SLF-I are likely to be secondary to structural or functional impairment of the pre/postcentral gyrus. In addition, the ALFF values of SLF were negatively correlated with the anxiety/somatization factor items, suggesting that ALFF values decreased as the patients' somatic discomfort increased. The previous study also reported that the ALFF values significantly decreased in the bilateral pre/postcentral gyrus in somatic MDD group compared to pure depression group13.
The CSF flow, blood CO2 concentration and the arteries around the brainstem are potential sources of physiological noise that can contaminate the BOLD signal in the brainstem14.15. The abnormal ALFF in MCP, which is immediately adjacent to the fourth ventricle, tending to noise rather than neuronal fluctuations.
The decreased ALFF regions in slow-5 band were located in the subcortical WM regions. It has been proposed that the "disconnection syndrome" between cortical and subcortical regions in MDD is caused by the WM alterations of cortical-subcortical networks16, and slow-5 band maybe a more sensitive frequency to the activity of cortical neurons17.14.18, these are consistent with our results. Previous rsfMRI study17showed that local separation characteristics of the slow-5 band are always greater than those of the slow-4 band, the slow-4 band component in typical band may mask the characteristics of the slow-5 band component, thus reducing the sensitivity of the classical band. Our study illustrated that slow-5 band may be a sensitive functional indicator for revealing the spontaneous brain activity in WM in the early stage of the disorder.
Conclusions
Our results indicated that abnormal WM spontaneous activity may be one of the pathophysiological mechanisms in MDD, which is associated with cognitive and emotional dysfunction and somatic discomfort in patients, and that functional damage to WM may precede structural damage in the early stage of disorder. Furthermore, Compared to the typical and slow-4 bands, slow-5 band may be a more sensitive functional indicator to detect the abnormal spontaneous brain activity in WM.Acknowledgements
We sincerely thank everyone who participated in our research.
References
1. Chaddock-Heyman L, Erickson KI, Voss MW, Powers JP, Knecht AM, Pontifex MB, et al. White matter microstructure is associated with cognitive control in children. Biol Psychol. (2013) 94:109–15. 10.1016/j.biopsycho.2013.05.008
2. Killgore WDS, Vanuk JR, Shane BR, Weber M, Bajaj S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol Dis. (2020) 134:104679. 10.1016/j.nbd.2019.104679
3. Bessette KL, Nave AM, Caprihan A, Stevens MC. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging Behav. (2014) 8:531-41. 10.1007/s11682-013-9274-8
4. Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. (2013) 38:49-56. 10.1503/jpn.110180
5. Hermesdorf M, Berger K, Szentkirályi A, Schwindt W, Dannlowski U, Wersching H. Reduced fractional anisotropy in patients with major depressive disorder and associations with vascular stiffness. Neuroimage Clin. (2017) 14:151-5. 10.1016/j.nicl.2017.01.013
6. Coloigner J, Batail JM, Commowick O, Corouge I, Robert G, Barillot C, et al. White Matter Abnormalities in Depression: A Categorical and Phenotypic Diffusion Mri Study. Neuroimage Clin (2019) 22:101710. 10.1016/j.nicl.2019.101710
7. Zhou G, Qin W, Zeng F, Liu P, Yang X, von Deneen KM, et al. White-Matter Microstructural Changes in Functional Dyspepsia: A Diffusion Tensor Imaging Study. Am J Gastroenterol (2013) 108:260-9. 10.1038/ajg.2012.405.
8. Zu M, Wang A, Bai T, Xie W, Guan J, Tian Y, et al. Resting-state functional connectivity between centromedial amygdala and insula as related to somatic symptoms in depressed patients: a preliminary study Psychosom. Med. (2019) 81:434-40. 10.1097/psy.0000000000000697
9. Klarborg B, Skak Madsen K, Vestergaard M, Skimminge A, Jernigan TL, Baaré WF. Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Hum Brain Mapp. 2013;34(12):3216-3232. doi:10.1002/hbm.22139
10. Urger SE, De Bellis MD, Hooper SR, Woolley DP, Chen SD, Provenzale J. The superior longitudinal fasciculus in typically developing children and adolescents: diffusion tensor imaging and neuropsychological correlates. J Child Neurol. 2015;30(1):9-20. doi:10.1177/0883073813520503
11. Biesbroek JM, Kuijf HJ, van der Graaf Y, et al. Association between subcortical vascular lesion location and cognition: a voxel-based and tract-based lesion-symptom mapping study. The SMART-MR study. PLoS One. 2013;8(4):e60541. Published 2013 Apr 8. doi:10.1371/journal.pone.0060541
12. Meinert S, Leehr EJ, Grotegerd D, Repple J, Förster K, Winter NR, et al. White matter fiber microstructure is associated with prior hospitalizations rather than acute symptomatology in major depressive disorder. Psychol Med. (2020) 52:1166-74. 10.1017/S0033291720002950
13. Liu P, Tu H, Zhang A, Yang C, Liu Z, Lei L, et al. Brain functional alterations in MDD patients with somatic symptoms: A resting-state fMRI study. J Affect Disord. (2021) 295:788-96. 10.1016/j.jad.2021.08.143
14. Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: Complex and reliable. Neuroimage. (2010) 49:1432. 10.1016/j.neuroimage.2009.09.037
15. Raitamaa L, Huotari N, Korhonen V, et al. Spectral analysis of physiological brain pulsations affecting the BOLD signal. Hum Brain Mapp. 2021;42(13) :4298-4313. doi:10.1002/hbm.25547
16. Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review of Diffusion Tensor Imaging Studies in Affective Disorders. Biol Psychiatry (2009) 66:814-23. 10.1016/j.biopsych.2009.05.024.
17. Xue SW, Li D, Weng XC, Northoff G, Li DW. Different neural manifestations of two slow frequency bands in resting functional magnetic resonance imaging:a systemic survey at regional, interregional, and network levels. Brain Connect. (2014) 4:242-55. 10.1089/brain.2013.0182
18. Buzs aki G, Draguhn A. Neuronal oscillations in cortical networks. Science. (2004) 304:1926–9. 10.1126/science.1099745
Figures