3028
Abnormal functional connectivity between white and gray matter is associated with hearing loss and cognitive dysfunction in presbycusis1School of Life Sciences, Tiangong University, Tianjin, China, 2Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China, 3Philips Healthcare, Shanghai, China, 4Vanderbilt University Institute of Imaging Science, Nashville, TN, United States
Synopsis
Keywords: White Matter, Aging
Presbycusis is characterized by high-frequency hearing loss and is closely related to cognitive decline. In this study, we aimed to explore the connectivity changes of WM and GM in the brains of presbycusis patients and their relationship with cognitive impairment and hearing loss. The results of this study indicated that the functional connectivity of WM and GM was abnormal in presbycusis patients, mainly in the PLIC, BCC, STG, par opercularis, etc. These changes might be associated with auditory processing and cognitive compensation. This study may provide new insights into the underlying neural mechanisms between presbycusis and cognitive impairment.Purpose
Presbycusis is one of the most common chronic diseases in the elderly population, mainly manifested as high-frequency hearing loss1. Previous studies have found that the structure and function of the gray matter (GM) have changed in the brains of presbycusis patients, and these changes were closely related to the decline of cognitive function2,3. However, the study of white matter (WM) in presbycusis patients is still in the exploratory stage. Based on functional magnetic resonance imaging (fMRI), this study uses a new data processing technology4,5 to explore the changes in functional connectivity of WM and GM in the brains of presbycusis patients and to establish a connection with hearing loss and cognitive dysfunction, providing a theoretical basis for clinical diagnosis.Methods
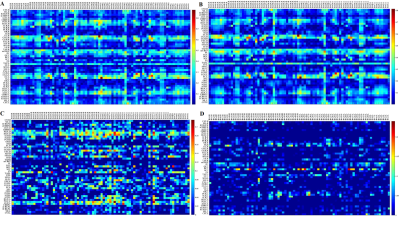
The study included 60 patients with presbycusis and 57 normal-hearing people with matched sex, age and education level as control. All subjects participated in tests for pure-tone thresholds and multiple cognitive domains. Resting-state fMRI was performed. By calculating the average hearing thresholds at 4 and 8 kHz, the high-frequency pure tone audiometry (H-PT) of both ears was obtained. The fMRI data were preprocessed to calculate the Pearson correlation coefficient (CC) between 48 WM tracts and 82 GM regions of each subject, and the two sample t-test between groups was performed. The CC with significant difference between groups after the correction was taken as the region of interest, and the correlation analysis was conducted with the behavioral scale score.Results
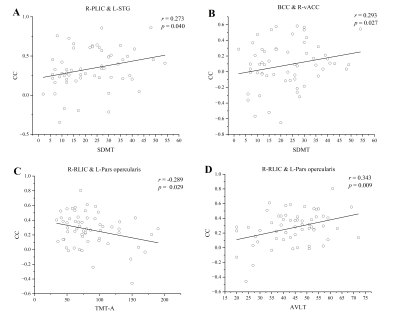
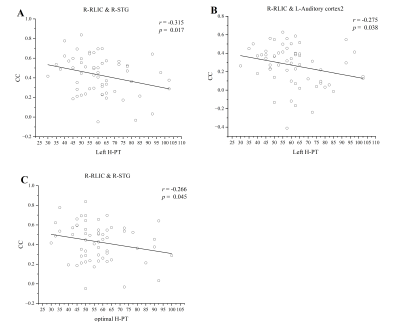
Compared with the healthy controls, the presbycusis patients had a general growth in CC between the WM and the GM regions. After FDR correction, the CCs between bilateral posterior limb of internal capsule (PLIC), right retrolenticular part of internal capsule (RLIC), superior fronto-occipital fasciculus and multiple gray matter regions were increased significantly in the presbycusis group. However, the CCs were decreased significantly between the body of corpus callosum (BCC) and the gray matter regions including dorsolateral prefrontal cortex, anterior cingulate cortex, somatosensory cortex, visual cortex. Notably, these changes were significantly correlated with the hearing thresholds and cognitive scale scores. The CC between the right PLIC and the left superior temporal gyrus (STG) was positively correlated with Symbol Digit Modalities Test scores (r = 0.273, p = 0.040), and the CC between the BCC and the right ventral anterior cingulate was positively correlated with Symbol Digit Modalities Test score (r = 0.293, p = 0.027). The CC between the right RLIC and the left pars opercularis was positively correlated with Auditory Verbal Learning Test score (r = 0.343, p = 0.009), but negatively correlated with Trail-Making Test A score (r = -0.289, p = 0.029). The CC between the right RLIC and the right STG was negatively correlated with H-PT of the left ear (r = -0.315, p = 0.017) and H-PT of the better ear (r = -0.266, p = 0.045). The CC between the right RLIC and the left secondary auditory cortex was negatively correlated with H-PT of left ear (r = -0.275, p = 0.038).Discussion
As the largest WM bundle in the human brain, the corpus callosum is a commissural fiber connecting the left and right cerebral cortex6. The posterior fibers across the posterior middle body, isthmus, and splenium transmit somatosensory, auditory, and visual information. It is reported that hearing loss can change the connection between the auditory-visual cortex and the auditory-somatosensory cortex7.We consider that the decrease of functional connectivity between the corpus callosum and the gray matter areas involved in sensorimotor, visual, and auditory processing may lead to the interruption of signal transmission, and thus affect the information integration and behavior regulation of them.The internal capsule contains most of the afferent auditory fibers within the cortex, which are closely related to auditory function. While most of the auditory fibers are located in the posterior limb of the internal capsule8,9. The STG and pars opercularis not only play an important role in auditory processing but also are related to various cognitive abilities. Analogous to the change of functional connectivity among GM brain regions, the enhancement of functional connectivity between WM tract and GM regions may be related to the compensation mechanism of cognitive function10. In this mechanism, the reduction of auditory information input may be compensated by enhanced cognitive resources. In addition, this study found that the increase in functional connectivity between the WM tract and GM regions was negatively correlated with H-PT. As described by the Information Degradation Hypothesis, as time go on, presbycusis patients may experience the redistribution of cognitive resources to auditory perception11.
Conclusion
Our results revealed that the functional connectivity of WM and GM in the brain of presbycusis patients has been changed, which may be associated with high-frequency hearing loss and cognitive impairment. In this study, the information transmission between the BCC and multiple GM areas in presbycusis patients was interrupted, which may provide support for the theory that hearing loss affects the connection between auditory cortex, visual cortex, and somatosensory cortex. After auditory deprivation, the functional connectivity between the internal capsule and the limbic system was enhanced, which may provide new insights into the compensation and resource redistribution mechanisms of hearing and cognition.Acknowledgements
This work was supported by the National Natural Science Foundation of China for Young Scholars (No. 81601479), Taishan Scholars Project (No. tsqn201812147).References
1. Panza F, Solfrizzi V, Seripa D, et al. Age-related hearing impairment and frailty in Alzheimer’s disease: interconnected associations and mechanisms. Frontiers in Aging Neuroscience. 2015, 7:113.
2. Ren F , Ma W , Li M , et al. Gray Matter Atrophy Is Associated With Cognitive Impairment in Patients With Presbycusis: A Comprehensive Morphometric Study. Frontiers in Neuroscience, 2018, 12.
3. Xing C, Chen Y, Tong Z, et al. Aberrant brains functional hubs and causal connectivity in presbycusis. Brains Imaging and Behavior, 2020, 15: 1-11.
4. Ding Z, Huang Y, Bailey S, et al. Detection of synchronous brains activity in white matter tracts at rest and under functional loading. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(3) : 595-600.
5. Li M, Newton A, Anderson A, et al. Characterization of the hemodynamic response function in white matter tracts for event-related fMRI. Nature communications, 2019, 10(1) : 1140.
6. Xue C, Shi L, Hui S, et al. Altered White Matter Microstructure in the Corpus Callosum and Its Cerebral Interhemispheric Tracts in Adolescent Idiopathic Scoliosis: Diffusion Tensor Imaging Analysis. AJNR. American journal of neuroradiology, 2018, 39(6) : 1177-1184.
7. Wang S, Chen B, Yu Y, et al. Alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study. Developmental Cognitive Neuroscience, 2019, 38.
8. Esen A, Aydin S, Bilgin B, et al. Microsurgical anatomy of the auditory radiations: revealing the enigmatic acoustic pathway from a surgical viewpoint. Journal of neurosurgery, 2022: 11-14.
9. Zhang Y, Zhang Z, Jia X, et al. Imaging Parameters of the Ipsilateral Medial Geniculate Body May Predict Prognosis of Patients with Idiopathic Unilateral Sudden Sensorineural Hearing Loss on the Basis of Diffusion Spectrum Imaging. AJNR. American journal of neuroradiology, 2020.
10. Cabeza R, Albert M, Belleville S, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature reviews. Neuroscience, 2018, 19(11) : 701-710.
11. Kate S, Christopher J, Helen E, et al. The Effects of Age-Related Hearing Loss on the Brains and Cognitive Function. Trends in Neurosciences, 2020, 43(10) : 810-821.
Figures