3026
Alterations of white matter connectivity in essential tremor with MR-guided focused ultrasound thalamotomy1Department of Radiology, Chinese PLA General Hospital, Beijing, China, 2Nankai University, Tianjing, China
Synopsis
Keywords: White Matter, Treatment, MRgFUS
This is important research to assess the effects of MRgFUS thalamotomy on white matter connectivity in ET. Results showed that MRgFUS might act the topologic properties on brain networks. Rich-club and small-world organizations exist in HC and ET. The right orbital part of the superior frontal gyrus and right putamen were identified as a hub in the ET group only, whereas the left putamen identified as hubs in the group only. Importantly, gamma and sigma correlated tremor improvement after MRgFUS thalamotomy, playing a role in reflecting tremor improvement for clinical treatment.Introduction
In recent years, the topologic properties of brain networks reveal the underlying pathophysiology and neurologic basis of essential tremor (ET). However, as a novel functional neurosurgical treatment, MR-guided focused ultrasound (MRgFUS) thalamotomy on the alteration properties of brain network is currently unclear. Based on previous experience with Parkinson’s disease (PD), MRgFUS could result in significant remodeling of brain structural networks [1]. Diffusion tensor imaging (DTI) has been used to assess white matter connectivity in the brains of individuals with ET. This approach has also been used to identify how the topologic organization of the brain network is affected by other neurological and psychiatric diseases, such as PD [2], Alzheimer’s disease [3], schizophrenia [4], autism [5], and progressive multiple sclerosis [6], etc. Therefore, in our study, we attempted to characterize the whole-brain connectivity in ET with MRgFUS by using DTI, which may help to reveal a potential network mechanism of MRgFUS in tremor improvement.Method
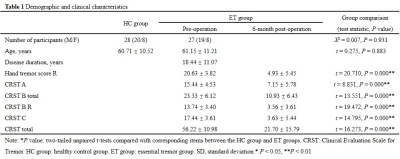
The study was approved by the ethics committees of the Chinese PLA General Hospital (ClinicalTrials.gov number: NCT04570046). All patients gave informed consent before MRgFUS thalamotomy. Twenty-seven ET patients were evaluated for tremor assessment with the clinical rating scale for tremor (CRST) and in the off-medication state preoperatively and at 6 months. Additional 28 age- and gender-matched healthy controls (HC) were recruited for comparison. The detailed demographic and clinical characteristics of the participants are summarized in Table 1. All participants were scanned on a 3.0-T MRI scanner (GE Health, Discovery 750). DTI was acquired with the following parameters: TR / TE 7522 / 80.8 msec; FOV 224 × 224mm; b values 1000sec/mm2; 112 × 112 matrix; 64 encoding directions; 2 mm isotropic resolution. Sagittal 3D T1-weighted images were obtained: TR / TE 6.656 / 2.928 msec; FOV 256 × 256mm; inversion time 800 msec; 256 × 256 matrix; Flip angle 7°; 1 mm thick slices; 192 contiguous slices. The DTI data were preprocessed by the PANDA toolbox (http://www.nitrc.org/projects/panda) based on FSL 5.0. The data processing steps were in accordance with previous studies [7]. T1 images were used for anatomical reference and to select the nodes of the brain network [8]. With this method, a 90 × 90 symmetric matrix was constructed for each participant. Rich club organization, efficiency properties and small-world properties were calculated by the GRETNA toolbox (http://www.nitrc.org/projects/gretna/). Statistical analyses of all data were performed using SPSS 26.0 statistical software (http://www.spss.com/).Result
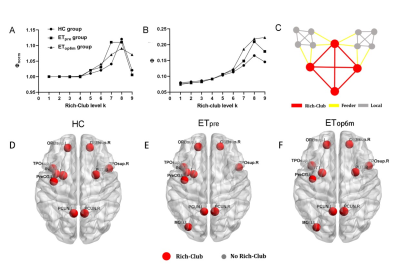
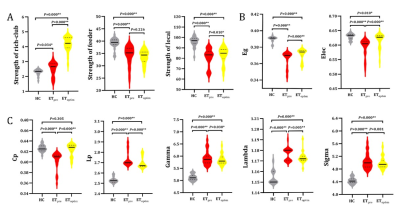
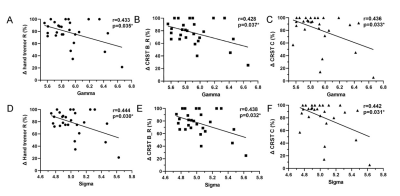
There were no significant differences in age, gender between HC group and group. Other demographic and clinical characteristics of the participant groups are displayed in Table 1. The nodes identified as hubs are shown in Figure 1. We found several common nodes that showed network hub properties for three groups ( (k) > 1 for a range of 5-9). Specifically, five consistent hub regions were identified in three groups: bilateral precuneus, orbital part of left superior frontal gyrus, left middle occipital gyrus, and left insula. However, the right orbital part of the superior frontal gyrus and right putamen were identified as a hub in the ET group only, whereas the left putamen identified as hubs in the group only (Fig.1). Figure 2 demonstrated that compared to that in the HC group, feeder and local connectivity strength significantly decreased whereas increased rich-club connectivity strength in the (P<0.05), (P<0.05), meaning that all patient groups had abnormal connections between rich-club organizations.Three groups also showed a small-world organization of white matter networks, as expressed by gamma larger than 1, lambda approximately 1, and sigma larger than 1(Fig.2). The group displayed a increased characteristic path length, global efficiency, and local efficiency, decreased clustering coefficient compared with the group (Fig.2; P < 0.001). We further calculated the correlation between graph metrics and tremor improvement, showing that gamma (Δ Hand tremor R: r = -0.433, P = 0.035; ΔCRST B_R: r = -0.428, P = 0.037;Δ CRST C: r = -0.436, P = 0.033) and sigma (Δ Hand tremor R: r = -0.444, P = 0.030; ΔCRST B_R: r = -0.438, P = 0.032;Δ CRST C: r = -0.442, P = 0.031) had a negative correlation with tremor improvement, respectively.Discussion
Rich-club organization may provide a new perspective on how MRgFUS affects brain topology and function. The main finding of this study was rich-club and local connectivity was significantly affected in ET after thalamotomy. Another main observation was that the brain regions with the connections involving rich club regions only exist in group without and HC group were distributed in the left putamen. Previous studies also reported that changes in functional imaging indicators (e.g., Functional connectivity, ALFF, nodal centrality) of the left putamen were associated with improved symptoms of tremor in PD [9-11]. Importantly, gamma and sigma had a negative correlation with tremor improvement (Fig. 4), indicating gamma and sigma have some implications for clinical practice.Conclusion
In summary, this is an important research to assess the effects of MRgFUS thalamotomy on white matter connectivity. MRgFUS might act the topologic properties on brain networks. Importantly, gamma and sigma correlated tremor improvement after MRgFUS thalamotomy, playing a role in reflecting tremor improvement for clinical treatment.Acknowledgements
This work has been supported by the National Natural Science Foundation of China (Nos. 81825012, and 82151309). Xin Lou is the author who received the funding.References
[1] J. Lin, X. Kang, Y. Xiong, D. Zhang, R. Zong, X. Yu, L. Pan, X. Lou, Convergent structural network and gene signatures for MRgFUS thalamotomy in patients with Parkinson's disease, NeuroImage 243 (2021) 118550.
[2] T. Liu, Y. Yan, J. Ai, D. Chen, J. Wu, B. Fang, T. Yan, Disrupted rich-club organization of brain structural networks in Parkinson's disease, Brain structure & function 226(7) (2021) 2205-2217.
[3] T. Yan, W. Wang, L. Yang, K. Chen, R. Chen, Y. Han, Rich club disturbances of the human connectome from subjective cognitive decline to Alzheimer's disease, Theranostics 8(12) (2018) 3237-3255.
[4] A. Schmidt, N.A. Crossley, F. Harrisberger, R. Smieskova, C. Lenz, A. Riecher-Rössler, U.E. Lang, P. McGuire, P. Fusar-Poli, S. Borgwardt, Structural Network Disorganization in Subjects at Clinical High Risk for Psychosis, Schizophrenia bulletin 43(3) (2017) 583-591.
[5] S.J. Hong, R. Vos de Wael, R.A.I. Bethlehem, S. Lariviere, C. Paquola, S.L. Valk, M.P. Milham, A. Di Martino, D.S. Margulies, J. Smallwood, B.C. Bernhardt, Atypical functional connectome hierarchy in autism, Nature communications 10(1) (2019) 1022.
[6] J.P. Stellmann, S. Hodecker, B. Cheng, N. Wanke, K.L. Young, C. Hilgetag, C. Gerloff, C. Heesen, G. Thomalla, S. Siemonsen, Reduced rich-club connectivity is related to disability in primary progressive MS, Neurology(R) neuroimmunology & neuroinflammation 4(5) (2017) e375.
[7] N. Shu, X. Wang, Q. Bi, T. Zhao, Y. Han, Disrupted Topologic Efficiency of White Matter Structural Connectome in Individuals with Subjective Cognitive Decline, Radiology 286(1) (2018) 229-238.
[8] M.P. van den Heuvel, O. Sporns, Rich-club organization of the human connectome, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(44) (2011) 15775-86.
[9] B. Shen, Y. Pan, X. Jiang, Z. Wu, J. Zhu, J. Dong, W. Zhang, P. Xu, Y. Dai, Y. Gao, C. Xiao, L. Zhang, Altered putamen and cerebellum connectivity among different subtypes of Parkinson's disease, CNS neuroscience & therapeutics 26(2) (2020) 207-214.
[10] Z. Wang, Y. Liu, X. Ruan, Y. Li, E. Li, G. Zhang, M. Li, X. Wei, Aberrant Amplitude of Low-Frequency Fluctuations in Different Frequency Bands in Patients With Parkinson's Disease, Frontiers in aging neuroscience 12 (2020) 576682.
[11] X. Suo, D. Lei, N. Li, W. Li, G.J. Kemp, J.A. Sweeney, R. Peng, Q. Gong, Disrupted morphological grey matter networks in early-stage Parkinson's disease, Brain structure & function 226(5) (2021) 1389-1403.
Figures