3022
Evidence of diffusion tensor imaging measures of white matter microstructure changes within six months after stroke1Lawson Imaging, Lawson Health Research Institute, London, ON, Canada, 2Department of Medical Biophysics, Western University, London, ON, Canada, 3Department of Clinical Neurological Sciences, Western University, London, ON, Canada, 4Department of Neurology and Neurosurgery, McGill University, Montréal, QC, Canada
Synopsis
Keywords: White Matter, Diffusion Tensor Imaging
White matter (WM) damage is associated with post-stroke cognitive impairment. In this study, we used diffusion tensor imaging (DTI) to investigate changes in WM microstructure in patients within 6-months following stroke. Quantitative DTI measurements were compared in patients at 6-months relative to 4-weeks post-stroke. DTI showed signs of reduced WM integrity in several WM tracts, including rostrum of corpus callosum and superior longitudinal fasciculus III in patients 6-months post-stroke. In elderly adults, WM microstructure can be disrupted following ischemic stroke. These findings motivate larger population stroke studies using advanced diffusion MRI techniques to further assess WM structure in stroke patients.INTRODUCTION
White matter (WM) damage secondary to vascular-related ischemic injury can occur in brains of older adults following stroke.1 Most vascular WM studies to date have reported fixed macroscopic WM injury (i.e. WM lesions) in stroke patients, with poor cognitive outcomes associated with severity of WM injury.1,2 However, less is currently known about vascular-related changes to the brain’s underlying WM microstructure and whether these microstructural changes could correlate better to post-stroke cognitive impairment. There is emerging evidence that microstructural WM breakdown, as assessed using diffusion tensor imaging (DTI), can occur prior to macroscopic WM lesions in elderly adults at risk for cognitive decline.3 This suggests that compared to macroscopic WM injury, microstructural WM disruptions may be earlier predictors of post-stroke cognitive impairment. Decreased fractional anisotropy (FA), increased mean diffusivity (MD), increased axial diffusivity (AD), and increased radial diffusivity (RD) are well reported DTI indicators of microstructural WM dysfunction in vascular diseases.4–6 However, DTI studies in stroke have been mostly limited to investigating the utility of FA to predict motor outcomes and clinical recovery after early acute phases of stroke.7,8 Thus, a more comprehensive investigation of DTI changes in cerebral WM within the months following stroke could shed further insight into the interaction between ischemic injury and WM-related cognitive impairment. In this study, we used DTI to assess longitudinal changes in WM microstructure in patients within 6-months following stroke to better understand which WM regions are sensitive to microstructural changes following an acute ischemic stroke.METHODS
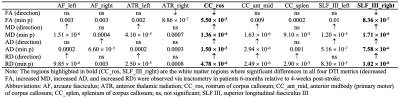
A 10-min diffusion-weighted imaging (DWI) brain scan was acquired in twelve stroke patients (4 females, age = 66-84 years) at 4-weeks post-stroke using a 3T hybrid PET/MRI scanner (Biograph mMR, Siemens Healthineers, Erlangen, Germany). DWI acquisition protocol: single-shot echo-planar imaging with 64 diffusion-encoding directions; b-values = 0 and 1000 s/mm2, 2 mm3 isotropic voxels. All twelve stroke patients were re-scanned at 6-months post-stroke to investigate longitudinal changes in cerebral WM microstructure following ischemic stroke. Each patient’s DWI data were preprocessed using an image analysis pipeline as previously described9 to generate FA, MD, AD, and RD maps. Tract-based spatial statistics (TBSS)10 and tractometry11 were used as previously described5,6 to conduct a within-group comparison of DTI indices (FA, MD, AD, RD) in cerebral WM in patients at 6-months versus 4-weeks post-stroke. Specifically, TBSS and tractometry were used to assess global and localized changes in WM tracts post-stroke, respectively. Tractometry was performed in nine WM bundles implicated in post-stroke cognitive impairment12,13 (see Table 1). All statistical analysis were corrected for multiple-comparisons using family-wise error (FWE), and p < 0.05 (FWE-corrected) was considered statistically significant.RESULTS
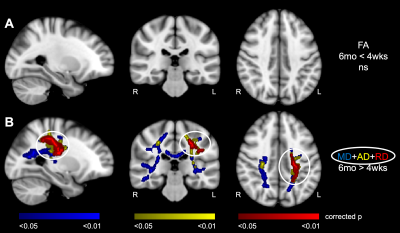
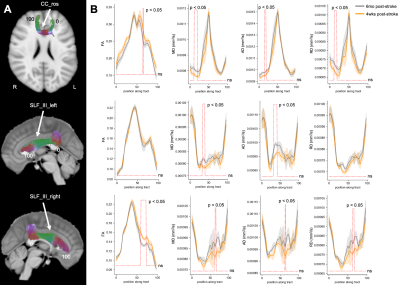
TBSS revealed diffuse patterns of increased MD, increased AD, and increased RD throughout brain WM of patients at 6-months relative to 4-weeks post-stroke (p < 0.05) (Figure 1). Interestingly, at 6-months post-stroke, overlapping WM clusters of increased MD, increased AD, and increased RD were observed in a few WM regions, including the left anterior thalamic radiation (ATR) and left superior longitudinal fasciculus (SLF). TBSS showed no significant differences in FA in WM of patients between 4-weeks and 6-months post-stroke. A summary of the tractometry findings in the stroke cohort is provided in Table 1, with tractometry plots for three representative WM bundles shown in Figure 2. Statistically significant along-tract differences in at least one DTI parameter (i.e. decreases in FA, increases in MD, increases in AD, or increases in RD), indicative of microstructural WM disruption, were observed in all nine WM bundles of patients at 6-months post-stroke (min p < 0.01). Importantly, significant changes in all four DTI metrics (decreased FA, increased MD, increased AD, and increased RD) were observed along tracts of rostrum of corpus callosum (CC) and right SLF III of patients 6-months post-stroke, suggesting microstructural integrity of these WM bundles is especially sensitive to post-stroke ischemic injury.DISCUSSION AND CONCLUSION
In this study, TBSS and tractometry of DTI indices revealed, in a small cohort, longitudinal changes in cerebral WM microstructure related to vascular injury within six months of an acute ischemic stroke. Using tractometry, we found that microstructural disruptions post-stroke were most prominent in rostrum of CC and SLF III, WM tracts associated with motor, visuospatial processing, and language function.13,14 Interestingly, tractometry detected subtle decreases in FA along a few WM tracts that were missed by TBSS, suggesting that tractometry may be more sensitive than TBSS to microstructural differences in WM following stroke. It should be noted that this study assessed post-stroke WM microstructure on a group-level, therefore no conclusions can be made regarding WM integrity in individual patients that are heterogeneous with respect to location of stroke infarct as well as impact of WM lesion(s) on WM structure and fiber reconstruction. Nevertheless, this study motivates larger sample stroke studies using advanced tractometry-based techniques, such as lesionometry15 to improve reconstruction and quantification of WM bundles around WM lesion(s) for further investigating WM microstructure and relationship of WM integrity to post-stroke cognitive impairment.Acknowledgements
This work was supported by funding from the Heart and Stroke Foundation of Canada Grants-in-Aid Award (UCA), Lawson Health Research Institute Internal Research Fund (UCA), and Canadian Institutes of Health Research (CIHR) Frederick and Charles Best Canada Graduate Scholarships Doctoral Award (SEP). The authors thank Professor Wolf-Dieter Heiss for supporting data collection through funding awarded to him through the Rolf M. Schwiete Stiftung funding at Max Planck Institute for Metabolism Research, Cologne, Germany.References
1. Cho A-H, Kim H-R, Kim W, et al. White Matter Hyperintensity in Ischemic Stroke Patients: It May Regress Over Time. J Stroke 2015;17:60–6.
2. Vermeer SE, Hollander M, van Dijk EJ, et al. Silent Brain Infarcts and White Matter Lesions Increase Stroke Risk in the General Population. Stroke 2003;34:1126–9.
3. van Leijsen EMC, Bergkamp MI, van Uden IWM, et al. Progression of White Matter Hyperintensities Preceded by Heterogeneous Decline of Microstructural Integrity. Stroke 2018;49:1386–93.
4. Qiao Y, He X, Zhang J, et al. The Associations Between White Matter Disruptions and Cognitive Decline at the Early Stage of Subcortical Vascular Cognitive Impairment: A Case–Control Study. Frontiers in Aging Neuroscience 2021;13.
5. Poirier SE, Suskin N, St. Lawrence KS, et al. Cardiac disease may exacerbate age-related white matter disruptions: improvements are feasible after cardiac rehabilitation. Proc Intl Soc Mag Reson Med 2021;29:0385.
6. Poirier SE, Suskin N, St. Lawrence KS, et al. Probing evidence of brain macrostructural disruptions in coronary artery disease: A diffusion MRI tractometry study. Proc Intl Soc Mag Reson Med 2022;30:3878.
7. Puig J, Blasco G, Daunis-I-Estadella J, et al. Decreased Corticospinal Tract Fractional Anisotropy Predicts Long-term Motor Outcome After Stroke. Stroke 2013;44:2016–8.
8. Feng W, Wang J, Chhatbar PY, et al. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Annals of Neurology 2015;78:860–70.
9. Poirier SE, Kwan BYM, Jurkiewicz MT, et al. 18F-FDG PET-guided diffusion tractography reveals white matter abnormalities around the epileptic focus in medically refractory epilepsy: implications for epilepsy surgical evaluation. European Journal of Hybrid Imaging 2020;4:10.
10. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006;31:1487–505.
11. Wasserthal J, Neher P, Maier-Hein KH. TractSeg - Fast and accurate white matter tract segmentation. NeuroImage 2018;183:239–53.
12. Urbanski M, Thiebaut de Schotten M, Rodrigo S, et al. DTI-MR tractography of white matter damage in stroke patients with neglect. Exp Brain Res 2011;208:491–505.
13. Li Y, Wu P, Liang F, et al. The Microstructural Status of the Corpus Callosum Is Associated with the Degree of Motor Function and Neurological Deficit in Stroke Patients. PLOS ONE 2015;10:e0122615.
14. Madhavan KM, McQueeny T, Howe SR, et al. Superior Longitudinal Fasciculus and Language Functioning in Healthy Aging. Brain Res 2014;1562:11–22.
15. Chamberland M, Winter M, Brice TAW, et al. Beyond Lesion-Load: Tractometry-Based Metrics for Characterizing White Matter Lesions within Fibre Pathways. In: Gyori N, Hutter J, Nath V, et al., eds. Computational Diffusion MRI. Cham: Springer International Publishing; 2021:227–37.Figures