3011
Diurnal Changes in Cerebrovascular Dynamics Measured from 4D-Flow1University of Wisconsin, Madison, Madison, WI, United States
Synopsis
Keywords: Neurofluids, Velocity & Flow, Aging
In this study, we investigated diurnal changes in cerebral hemodynamics from 4D-Flow in a large population of cognitively healthy older adults and in a younger group of healthy volunteers. To separate physiological and technical variability of 4D-Flow measures, volunteers were scanned at 7am, 4pm, and 10pm on the same day three times for each timepoint. Data supports strong cerebral blood flow fluctuations of physiological origin that are much higher than the technical variability.Introduction:
4D-Flow MRI is a non-invasive technique that can be used to provide comprehensive characterization of cerebral hemodynamic fluctuations, including measures of cardiac pulsations and vasomotion that are hypothesized to drive glymphatic flow and waste clearance. 4D-Flow studies have identified altered vascular dynamics in Alzheimer’s subjects; however, hemodynamic variability in cognitively normal individuals has not been well established.1,2 Circadian rhythms can lead to hemodynamic changes and have been implicated to aid in brain metabolite waste clearance.3 Further, disruptions of circadian rhythms have been associated with various hallmark proteinopathies of common neurological disorders.4 Therefore, the purpose of this study was to improve our understanding of the technical and diurnal physiological variations in 4D-Flow vascular measures in cognitively normal participants. This was achieved through retrospective analysis and prospective test-retest scanning of subjects throughout the day.Methods:
Subjects: A data repository of 750 cognitively normal older adults was studied.5 In that dataset, 572 participants were scanned in the morning (<12pm) and 158 in the afternoon (>12pm). To control for the effects of age and sex, 158 participants from the morning group were propensity score matched to the afternoon group (morning 67 ± 8yrs, 115f ; afternoon 67 ± 8yrs, 113f).6 In addition, 7 young healthy volunteers were recruited (26±4y, 2F) for prospective, repeated studies. MRI: Neurovascular 4D-Flow data were acquired on 3.0T scanners (GE Healthcare) with a 3D radial sequence.7 Imaging parameters included: Venc = 80cm/s, imaging volume = 22x22x16cm3, 0.75mm3 isotropic resolution, TR/TE = 7.8/2.2ms and scan time ~5.6min. Image reconstruction of 20 cardiac phases was performed retrospectively.8 Data were background phase and velocity aliasing corrected.9 In prospective studies, participants were scanned at 7am, 4pm, and 10pm. At each time point, 3 scans were performed with the participant taken out of the room after the first scan and repositioned for two consecutive scans. Flow measurements were performed in MATLAB (Mathworks, Natick, MA) on the dynamic data using a semi-automated centerline process that extracted the vascular tree.10 Dynamic blood flow was quantified in the internal carotid (ICA), basilar (BA) and middle cerebral arteries (MCA). Pulsatility index (PI) was calculated from flow curves: (Qmax-Qmin)/Qmean, Q=flow. Non-parametric Wilcoxon rank sum and signed rank tests were used to study group differences (statistically significant P<0.05). A stepwise regression linear model was used to determine explanatory predictor variable effects on the larger data repository sample. Predictor variables included MRI time of day, fasting time, heart-rate, sex, and age. Outcome variables were total cerebral blood flow (TCBF) (ICAs+BA) and cerebral pulsatility index in the ICAs and MCAs. Linear correlation and Bland-Altman analyses including Pearson and repeatability coefficients were calculated to characterize young volunteers within and across session variability.Results:
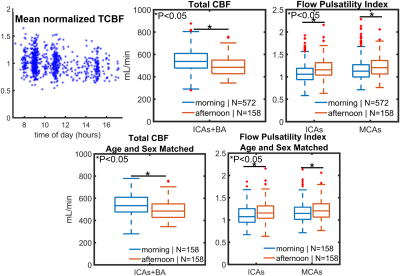
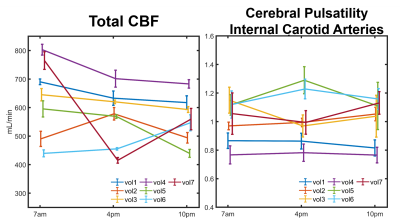
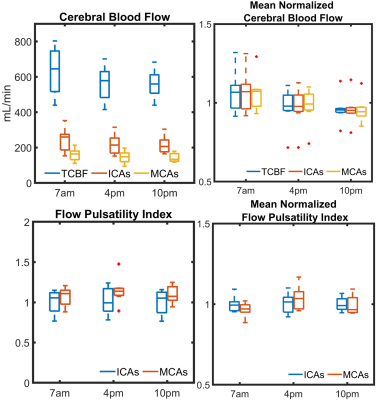
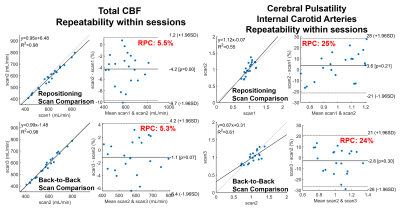
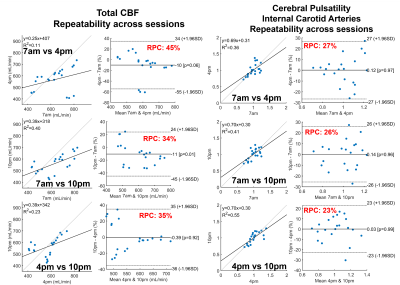
Significantly higher TCBF and lower cerebral pulsatility were observed in participants scanned in the morning compared to those scanned in the afternoon even after matching for age and sex (Fig. 1). Stepwise regression analysis on the complete 750 participant sample showed age and time of MRI as the only co-variates that significantly explained TCBF (P<0.001, R2=0.14) and cerebral pulsatility (P<0.001, R2=0.38). Young volunteers exhibited throughout-day variability; TCBF decreased with time of day in most volunteers (Fig. 2). Box plots summarizing volunteer data showed a similar trend to observations in the large data repository with decreasing TCBF and increasing cerebral pulsatility in the afternoon (Fig. 3). Inter-session mean normalization revealed very low variability in TCBF at 10pm. Intra-session linear correlation and Bland-Altman analysis demonstrated low levels of variability in TCBF measures in both repositioning and back-to-back scan conditions, with Pearson and repeatability coefficients of 0.98 and 5.5% respectively (Fig. 4, left column). Cerebral pulsatility measures in the ICAs were more variable with Pearson and repeatability coefficients of ~0.58 and ~25% respectively (Fig. 4, right column). Inter-session comparisons (Fig. 5) showed high levels of variability in TCBF measures (Pearson coeff. ~0.24 and repeatability coeff. ~38%) (left column); however, inter- and intra- session cerebral pulsatility variability were very similar (Pearson coeff. ~0.44 and repeatability coeff. ~0.25%) (right column).Discussion and Conclusions:
Significant diurnal cerebral hemodynamic variability was observed in cognitively healthy older participants. Morning scan sessions were associated with larger perfusion and lower cerebral pulsatility, even after age and sex matching. In prospective volunteer experiments, TCBF mostly decreased throughout the day. Test-retest data including repositioning and back-to-back conditions showed low levels of TCBF variability (repeatability coefficient ~5%) demonstrating high intra-session repeatability. However, throughout-day TCBF variability was high (repeatability coefficient ~38%) likely due to circadian rhythms and neurovascular coupled metabolic demand. Cerebral pulsatility displayed similar levels of variability within and across sessions (both repeatability coefficients ~25%), with higher within session but lower across session variability compared to TCBF. This finding suggests that in young volunteers cerebral pulsatility is less sensitive to daily metabolic changes and more related to vascular function. In this study, we have observed physiological cerebral hemodynamic fluctuations throughout the day in older and younger participants. Highly reproducible cerebral blood flow measurements support the conclusion that fluctuations are from physiological origin. Results indicate time of scan needs to be included in multi-variable regression analysis of cerebrovascular and perfusion studies.Acknowledgements
We gratefully acknowledge research support from GE Healthcare, and funding support from the Alzheimer's Association (AARFD-20-678095) and from NIH grants R01AG075788, R21AG077337, R01AG021155, P30AG062715, UL1TR002373, and F31AG071183.References
1. Rivera-Rivera LA, Eisenmenger L, Cody KA, et al. Cerebrovascular stiffness and flow dynamics in the presence of amyloid and tau biomarkers. Alzheimers Dement (Amst) 2021; 13: e12253–e12253.
2. Conroy DA, Spielman AJ, Scott RQ. Daily rhythm of cerebral blood flow velocity. J Circadian Rhythms 2005; 3: 3.
3. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342: 373–377.
4. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science 2020; 370: 50–56.
5. Roberts GS, Peret A, Jonaitis EM, et al. Proc. Intl. Soc. Mag. Reson. Med. 30 (2022) #0007
6. Ho D, Imai K, King G, et al. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software; Vol 1, Issue 8 (2011), https://www.jstatsoft.org/v042/i08 (2011).
7. Johnson KM, Lum DP, Turski PA, et al. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magnetic Resonance in Medicine 2008; 60: 1329–1336.
8. Liu, J, Redmond MJ, Brodsky EK, et al. Generation and visualization of four-dimensional MR angiography data using an undersampled 3-D projection trajectory. IEEE Transactions on Medical Imaging 2006; 25: 148–157.
9. Loecher M, Schrauben E, Johnson KM, et al. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J Magn Reson Imaging 2016; 43: 833–842.
10. Schrauben E, Wåhlin A, Ambarki K, et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. Journal of magnetic resonance imaging : JMRI 2015; 42: 1458–1464.
Figures