3004
Associations between Extracellular Diffusivity Along Perivascular Space (eALPS) and Structural Abnormalities in Brain
Sang-Young Kim1, Eunju Kim1, Jinwoo Hwang1, Joo Hyun Kim1, and Chae Jung Park2
1Health Systems, Philips Healthcare, Seoul, Korea, Republic of, 2Department of Radiology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea, Republic of
1Health Systems, Philips Healthcare, Seoul, Korea, Republic of, 2Department of Radiology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea, Republic of
Synopsis
Keywords: Neurofluids, Diffusion Tensor Imaging
Non-invasive measure of glymphatic function or flow in vivo has gained a great attention from neuroscience research community. Diffusion tensor imaging (DTI) can provide a mean that measures the diffusivity along perivascular space (ALPS). However, conventional DTI captures diffusion indices from both tissue and free water compartments due to partial volume effects, which may raise a concern about validity of DTI-ALPS index for glymphatic function. In this work, we present a novel method that can extract extracellular diffusivity (i.e., eALPS) using free water eliminated DTI and investigate the relationship between eALPS and structural abnormalities in brain.INTRODUCTION
Since glymphatic system, known as brain waste clearance pathway by cerebrospinal fluid (CSF) microcirculation, was first discovered by two-photon microscopy study in mice brain,1 much attention has been paid to non-invasive MR-based measurement of human brain in vivo. One of straightforward method includes glymphatic MRI which allows direct visualization of CSF transport of gadolinium (Gd)-based contrast agent following intrathecal or intravenous administration.2 However, the method requires repetitive MR measurements at multiple time points, thus clinical applicability is limited. Another promising technique is a diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluation of glymphatic function.3 Given that DTI data are readily available in clinical practice, DTI-ALPS has been widely applied to patients with various neurological disorders. Moreover, recent study demonstrated that the ALPS index had significant negative associations with glymphatic MRI after intrathecal administration of Gd-based contrast agent.4 It should be noted that conventional DTI captures diffusion indices from both tissue and free water compartments such as CSF or edema, due to partial volume effect in an image voxel. Thus, the ALPS index computed from mixed environments (e.g., tissue + free water) may not be specific to the diffusivity along perivascular space (PVS) where diffusion is unrestricted in microscale. In this work, we resolve this problem by utilizing free water eliminated DTI (FWE-DTI)5,6 and propose a modified ALPS index tuned to measure extracellular diffusivity along PVS (eALPS). Furthermore, we evaluate the associations between eALPS index and structural abnormalities in brain.METHODS
Twenty objectively-cognitively normal older adults (age > 65 years) with or without subjective cognitive decline were included in this retrospective study. MRI data were acquired using a 3T MRI scanner (Ingenia Elition X or Ingenia CX, Philips Healthcare, Best, the Netherlands) with 32-channel dS head coil. The 3D T1-weighted images were acquired using 3D FFE sequence with following parameters: 3D T1w - TR/TE=4.5/2.0 msec; FA=8°; 1 mm isotropic resolution. And DTI data were acquired using single-shot spin-echo EPI sequence with following parameters: b-value = 0 and 1000 s/mm2; diffusion directions = 32; 2 mm isotropic resolution. The preprocessing pipeline for anatomical T1w and DTI data is illustrated in Figure 1. The preprocessed T1w data were used for morphometric measurements of structure volumes (e.g., choroid plexus (CP) and lateral ventricle) using FreeSurfer (ver. 6.0).7 And the extracted volumes of CP and lateral ventricle were normalized to estimated total intracranial volume (eTIV). After preprocessing of DTI, conventional DTI fitting were conducted in original diffusion space using DIPY library. To fit the FWE-DTI model to single-shell data, regularized gradient descent (RGD) algorithms with hybrid initialization method6 (i.e., initialization based on b0 and tissue’s mean diffusivity) were utilized to obtain plausible parameter maps. The tensor maps computed from conventional DTI and FWE-DTI were non-linearly registered to 2 mm isotropic MNI template followed by reorienting tensors to account for changes in orientation as well as displacement of location, prior to automatic calculation of ALPS index. We speculate that extracellular diffusivity can be estimated by subtracting intracellular diffusivity for FWE-DTI from mixed intra- and extracellular diffusivity for conventional DTI. Thus, eALPS index can be easily computed from average values of diffusivities along left-right directions (x-axis) in the same ROIs for conventional ALPS method (eALPS = mean (Dxx,proj, Dxx,assoc)).RESULTS
Basic idea to measure extracellular diffusivity in brain is shown in Figure 2. The free water compartment determined from FWE-DTI represents water molecules that are not restricted, thus representing extracellular space. This is evident in Figure 2-(C) showing no directional dependence of diffusion with respect to applied diffusion gradients, indicating that the calculation of eALPS index does not require normalization to diffusivity perpendicular to PVS (i.e., y- and z-axis diffusion). Interestingly, we found that the eALPS index computed from extracellular diffusivity maps was positively correlated with the volumes of CP (p=0.04, Pearson correlation coefficient r=0.449) and ventricles (p=0.02, r=0.486) normalized to eTIV, but there were no significant associations between conventional DTI-ALPS index and those volumes (p>0.05) (Figure 3). Of note, there was a trend of negative association between eALPS and DTI-ALPS index, but it did not reach to significance level, possibly due to small sample size. As an exploratory analysis, we attempted to see further relationship of eALPS index with free water volume fraction over the whole brain. The general linear model revealed that the eALPS index was positively associated with free water volume fraction in widespread white matter and third ventricle as well as the around of cerebral artery (Figure 4).DISCUSSION AND CONCLUSION
In this work, we proposed a modified measure of conventional DTI-ALPS, called eALPS index for noninvasive measurement of glymphatic function. Strikingly, the eALPS index had positive associations with CP and ventricle volumes. And it is of worth to note that the eALPS index had strong positive correlation with free water volume fraction volume, suggesting that glymphatic function might be associated with free water volume in brain. However, in order to elucidate our finding, further study with larger sample size would be warranted. In conclusion, the eALPS index can be an alternative measure for the assessment of glymphatic function in vivo and might be more sensitive to pathological changes in brain.Acknowledgements
No acknowledgement found.References
- Iliff JJ, Wang M, Liao Y et al., A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111
- Illiff JJ, Lee H, Yu M et al., Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013;123:1299-309.
- Taoka T, Masutani Y, Kawai H et al., Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI‑ALPS) in Alzheimer’s disease cases. Jpn J Radiol 2017;35:172-178.
- Zhang W, Zhou Y, Wang J et al., Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage 2021;238:118257.
- Pasternak O, Sochen N, Gur Y et al., Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009;62:717-30.
- Golub M, Henriques RN and Nunes RG. Free-water DTI estimates from single b-value data might seem plausible but must be interpreted with care. Magn Reson Med 2021;85:2537-2551.
- Fischl B, Salat DH, Busa E., Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341-355.
Figures
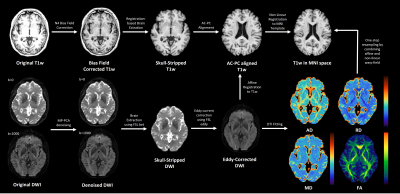
Figure
1. Preprocessing pipeline for anatomical
T1w and diffusion MRI
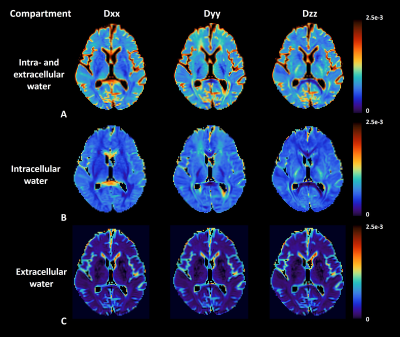
Figure
2. Diffusivity maps from diffusion tensor.
(A) Conventional and (B) free water eliminated diffusivity maps are shown. (C)
Extracellular diffusivity maps are calculated by subtracting tissue diffusivity
from conventional DTI diffusivity. Note that there are no directional dependences
in brain parenchyma on extracellular diffusivity maps, indicating isotropic
diffusion.
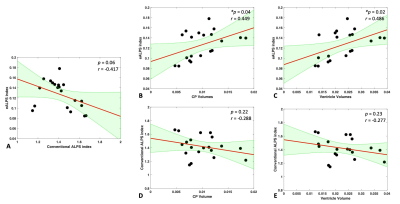
Figure
3. Relationships between ALPS index
(conventional and eALPS) and the volumes of choroid plexus (CP) and ventricle
(lateral + 3rd + 4th). (A) There is a trend of negative
association between conventional ALPS and eALPS index, but it does not reach
significance level. (B, C) There are significant positive associations between
eALPS index and CP and ventricle volumes. (D, E) No significant associations
are observed between conventional ALPS index and structural volumes.
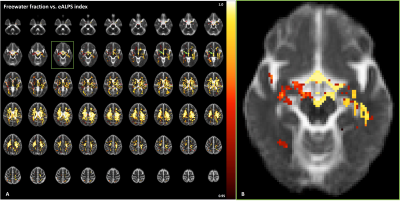
Figure
4. Results of voxel-wise general linear
model (GLM) fitting to evaluate relationship between free water volume fraction
and eALPS index. (A) Statistically significant voxels are displayed in
red-yellow (1-p value), overlaid onto average free water fraction map
from all subjects. Note that the eALPS index is positively associated with free
water volume fractions in widespread white matter, 3rd ventricle
(green arrow) and (B) the around of cerebral artery. Significance was set at p
< 0.05 and was corrected for multiple comparisons with family-wise
error.
DOI: https://doi.org/10.58530/2023/3004