2999
Intrinsic CSF outflow declines with age in healthy humans detected by spin-labeling MRI
Vadim Malis1, Won Bae1,2, Asako Yamamoto3, Linda McEvoy1, Marin McDonald1, and Mitsue Miyazaki1
1Radiology, University of California, San Diego, La Jolla, CA, United States, 2VA San Diego Healthcare System, San Diego, CA, United States, 3Radiology, Teikyo University, Tokyo, Japan
1Radiology, University of California, San Diego, La Jolla, CA, United States, 2VA San Diego Healthcare System, San Diego, CA, United States, 3Radiology, Teikyo University, Tokyo, Japan
Synopsis
Keywords: Neurofluids, Aging, cerebrospinal fluid (CSF), intrinsic CSF outflow, egress pathways
Parasagittal dura (PSD) along the superior sagittal sinus (SSS) can be visualized using FLAIR and 3D SSFSE without gadolinium-contrast administration. Visualization of intrinsic CSF outflow from the PSD to the SSS can be achieved in human brains using spin-labeling MRI at clinical 3-T MRI. The spin-labeling MRI shows intrinsically tagged CSF outflow from the upper PSD and lower PSD to SSS. The quantitative intrinsic CSF outflow metrics indicate an age-related decline of the intrinsic CSF outflow at the SSS in human brains.Introduction
Clearance of CSF outflow is important for the removal of toxins from the brain to maintain healthy brain and prevents for neurogenerative diseases. However, the egress pathway of the egress and quantitative measures of intrinsic CSF drainage remain unknown. In general, glymphatic studies were performed in small animals with invasive tracers [1,2]. Recently, MRI using intrathecal and intravenous administrations of gadolinium-based contrast agents (GBCA) as a tracer show dural lymphatic vessels [3-5]. The purpose of this study is to develop non-contrast MRI techniques to depict the intrinsic CSF outlet, measure their quantitative metrics, and investigate an age effect in human brain.Methods
The study was approved by the Institutional Review Board. All MR imaging data were obtained with a clinical 3-T MR imager (Vantage Galan 3T, Canon Medical Systems, Japan) in healthy subjects without neurodegenerative diseases (10 males and 6 females; mean age, 47.6 ± 18.9 years; range, 19-71 years) who had given written informed consent. For localization of superior sagittal sinus (SSS), upper parasagittal dura (PSD), and lower PSD [5], T2- FLAIR and 3D centric ky-kz single-shot fast spin echo (3D cSSFSE) sequences were optimized for the contrast. For 4D time-resolved MRI, both labeled and control were acquired and subtracted for quantitative analysis and visualization of intrinsic CSF outflow[6].Results
We have developed MRI techniques without administration of GBCA to optimize the contrast of PSD and SSS in meninges. Figure 1 shows the dural lymphatic vessels by tracer study, our proposed dura mater pathway, and another possible pathway. The subtracted color maps of contiguous slices show marked CSF moved toward the SSS. Using spin-labeling MRI, we revealed that tagged CSF from PSD purged into the SSS, as shown in Figure 2. In Figure 3, quantitative CSF outflow metrics show a decline of the fluid outflow at the SSS in human brains of older individuals. Furthermore, we observed a sharp decline of the outflow after over 60 years, with a significant correlation between young (19 to 59 years) and old (60 to 71 years) (r = 0.82, P < 0.001).Discussion
Based on our present results, intrinsic CSF outflow may occur in two steps: first with CSF egress from the SAS to the compartmentalized fluid at the dura mater and PSD, and then from the PSD into the SSS. Given that quantitative outflow measurements of healthy younger and older subjects show significant difference in outflow values, the present study offers hope in the establishment of a normative values for CSF outflow which then can be used to shed light on the potential role of the glymphatic system in the causative pathway of neurodegeneration in human brain.Conclusions
Our study demonstrates unambiguous visualization of intrinsic CSF outflow at the SSS, and quantitative measures indicate a decline of CSF outflow metrics with age. The decline of CSF outflow may be a significant factor in the age-related prevalence of neurodegenerative diseases.Acknowledgements
This work was supported by an NIH grant RF1AG076692 (M.M.) and a grant by Canon Medical Systems, Japan (35938).References
1] Nedergaard M. Science 2013;340:1529–1530.
2] Iliff JJ, Wang M, Zeppenfeld DM,et al. Journal of Neuroscience 2013;33:18190–18199.
3] Absinta M, Ha SK, Nair G, et al. eLife 2017;6:e29738.
4] De Mesquita S, Fu Z, and Kipnis J. Neuron 2018;24:375–388.
5] Ringstad G, and Eide PK. Nature Communications 2020;11:354.
6] Malis V, Bae W, Yamamoto A, McEvoy KL, McDonald MA, Miyazaki M. MRMS in press.
Figures
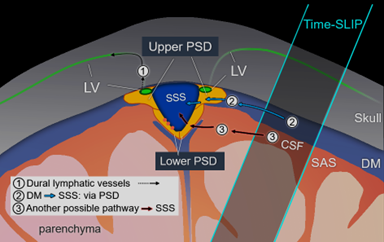
Fig. 1 Drawing of possible egress pathways of CSF. (1) Pathway of egress through dural lymphatic vessels, observed using
tracer studies, including gadolinium-based contrast agent. (2) The pathway of
CSF from the dura mater (DM) to parasagittal dura (PSD), and then to SSS,
observed using our tagged CSF method. (3) Another possible pathway where we
observe in some individuals. PSD was divided in upper PSD and lower PSD.
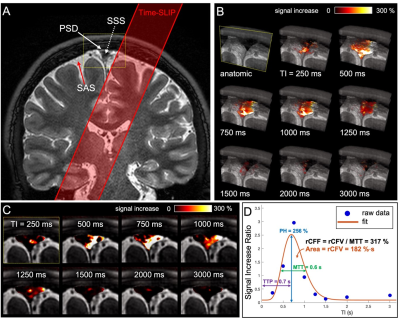
Fig. 2 A) Coronal 3D cSSFSE image with a Time-SLIP pulse (red
box). B) A series of oblique 3D spin labeling images with various
inversion times (TIs) fused over 3D cSSFSE image, enlargement of meninges area
in A). C) Straight coronal fusion images of intrinsic CSF signals around
the SSS region over 3D cSSFSE images at various TIs show tagged fluid outflow
from the tag pulse to dura mater and PSD into the SSS. D) Tagged CSF
outflow signal at the SSS. Circles show the data points, and the line indicates
the curve fit.
Fig. 3 A) CSF outflow of the entire SSS
region on 16 participants between 19 and 71 years show a decline of intrinsic
CSF outflow with age, especially over 60 years. B) Tagged CSF
outflow rate of entire SSS in two groups, with markedly decreased in the older
group. C) Quantitative CSF outflow metrics between the two groups. PH, rCFV,
and rCFF were significantly different between the two groups.
DOI: https://doi.org/10.58530/2023/2999