2994
CEST effect of dimethyl sulfoxide (DMSO) at negative offset frequency1Department of Biomedical Engineering, City University of Hong Kong, Kowloon, Hong Kong, 2Hong Kong Centre for Cerebro-Cardiovascular Health Engineering (COCHE), New Territories, Hong Kong, 3Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4City University of Hong Kong Shenzhen Research Institute, Shenzhen, China, 5Tung Biomedical Sciences Centre, City University of Hong Kong, Kowloon, Hong Kong
Synopsis
Keywords: CEST & MT, Molecular Imaging
CEST MRI can detect mM range of endogenous and exogenous molecules, and bound small molecules such as glycogen and lactate via rNOE. Here we reported for the first time that DMSO and its structural analogs in aqueous solution had distinctive CEST peaks at the negative offsets of Z-spectrum. CEST effect of DMSO was dependent on saturation power and concentration, and less sensitive to tested temperature. Alcohols, acetone, acetonitrile, acetic acid and N,N-dimethylformamide also showed observable CEST peaks at the negative offsets. Interaction between DMSO and water molecule, such as hydrogen bonding, could contribute to the observed CEST effect.Introduction
Dimethyl sulfoxide (DMSO) is a common organic solvent, pharmacological agent and cryoprotectant. Understanding its interaction with biomolecules in aqueous environment is essential for the DMSO usage in biomedical applications. We reported for the first time that DMSO in aqueous solution was detectable with Chemical Exchange Saturation Transfer Magnetic Resonance Imaging (CEST MRI) at around -2 ppm. The positive offset frequencies have been widely studies, including amide proton transfer (APT) at 3.5 ppm 1, creatine at 1.8 ppm 2, and glucose at 1-3 ppm 3. For the negative side of Z-spectrum, relayed nuclear Overhauser effect (rNOE) where non-exchangeable aliphatic protons transfer magnetization to neighboring dipolar-coupled protons which then exchange with water, is known to indicate macromolecules such as glycogen 4 and lipid contents 5, and bound small molecules such as lactate 6 and caffeine7. It is a new finding to observe the CEST effect of DMSO at a negative offset, which could be attributed to the intermolecular hydrogen bonding between DMSO and water8,9. We performed CEST experiments on DMSO and structurally similar polar organic solvents to investigate the origin of the observed CEST peak.Materials and methods
ACS grade DMSO, N,N-dimethylformamide (DMF) and acetic acid were purchased from Sigma-Aldrich. ACS grade acetone, methanol, and acetonitrile were purchased from Anaqua. Ethanol and isopropyl alcohol were purchased from UNI-Chem and Oriental Chemicals and Lab Supplies.Phantom solutions were prepared by mixing DMSO or structural analogs in deionized water to final concentrations of 2, 5, 10, 20 v/v%. Samples then were transferred to 0.5 mL tubes for image acquisition. Unless otherwise specified, CEST image acquisition was performed at 37 °C using a horizontal bore 3T preclinical scanner (Bruker, Ettlingen, Germany) with continuous-wave pulse, RARE factor = 32, TR = 9000 ms, TE = 75 ms, FOV = 35x35 mm2, saturation duration (Tsat) = 4 s, and image size = 64x64. Z-spectra were acquired at B1 amplitudes of 0.4, 0.8, 1.2, 1.5, and 2.0 μT, with a saturation offset range of -10 to 10 ppm. Offset step size of 0.1 ppm was used between -2 and 2 ppm and 0.2 ppm step size for rest of the offset ranges. Z-spectrum was processed with direct water saturation removal by Lorentzian fitting, using our customized Matlab (Mathworks, Massachusetts, USA) codes. Exchange rate (kex) was calculated with quantification of exchange rate using varying saturation power (QUESP) experiment10,11.
Results
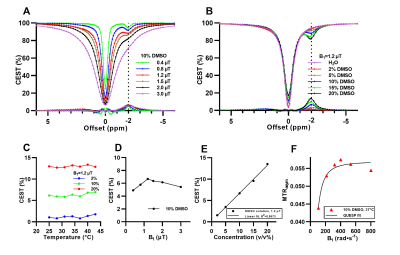
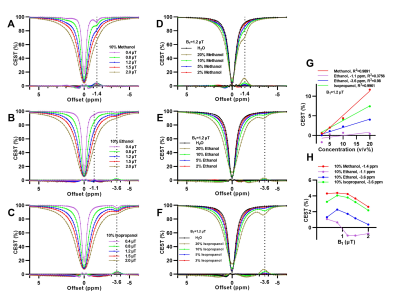
DMSO solutions at various volume ratios were imaged. 10 v/v% DMSO showed a distinctive dip at around -2 ppm away from water with ~6% CEST contrast (Fig. 1A). The maximum CEST contrast of ~13.5% was observed at 20 v/v% with B1=1.2 μT (Fig. 1B). It was dependent on B1 amplitude and DMSO concentration, and was less dependent on the tested temperature (25-44 °C) (Fig 1C-E). QUESP fit for 10 v/v% DMSO at 37 °C was plotted in Fig. 1F, with estimated kex around 60 Hz. Structural analogs of DMSO, such as alcohols, acetone, acetic acid, and N,N-dimethylformamide, also showed noticeable CEST peaks at negative frequency offsets, with B1 and concentration dependencies (Fig. 2 and Fig. 3). CEST contrast were observed at -1.4 ppm for methanol, at -1.1 ppm and -3.6 ppm for ethanol, and -3.6 ppm for isopropanol (Fig. 2). Moreover, DMF, acetone, acetonitrile and acetic acid all generated CEST contrast at a range of -1.8 ppm to -2.8 ppm (Fig 3).Discussion
Aliphatic protons in macromolecules can transfer saturation to nearby exchangeable protons (such as those of hydroxyl groups or bound water) both intermolecularly and intramolecularly through NOE and generate CEST contrast at negative offsets5,12,13. From our phantom experiment results, CEST peak of DMSO could have an origin similar to rNOE signals in the presence of methyl groups, leading to the observed CEST contrast ~ 6.5% at around -2 ppm for 10 v/v% DMSO (Fig 1). Spectroscopy and molecular dynamic studies indicate that hydrogen bonding between DMSO and water at low concentration could alter nearby water structure8,9. To further illustrate the concept, structural analogs of DMSO were selected since they all have methyl group and electronegative group. Polar protic solvents such as methanol, and polar aprotic solvents such acetone and acetonitrile are also known to have the capability to interact with neighboring water9. These solvents generated CEST contrast at a range of -1.1 ppm to -3.6 ppm, and also showed B1 and concentration dependencies (Fig. 2, Fig. 3). The intermolecular hydrogen bonding between these polar solvents and water might be one source of the detected peaks at negative offsets. For alcohols and acetic acid, CEST effects at negative side were also observed although having exchangeable hydroxyl groups in their structures. Further experiments for investigating the mechanism behind the observed peaks are underway.Conclusion
DMSO its analogs generated CEST contrast at the negative offsets, which is conventionally known as NOE. Here, we demonstrated that in additional to aliphatic protons from macromolecules, small, organic polar molecules solvated in water at measured volume ratios also generated distinctive CEST signals at negative offsets. This could be due to the hydrogen bonding between these molecules and water. We are now examining the exchange properties and related contrast mechanisms of these common solvents.Acknowledgements
Authors would like to acknowledge the funding supports from Research Grants Council (11102218, PDFS2122-1S01, 11200422, RFS2223-1S02, C1134-20G); City University of Hong Kong (7005433, 7005626, 9239070, 9609307, 9610560); National Natural Science Foundation China (81871409); Tung Biomedical Sciences Centre; Hong Kong Centre for Cerebro-cardiovascular Health EngineeringReferences
[1] Zhou, Jinyuan, Jean-Francois Payen, David A. Wilson, Richard J. Traystman, and Peter van Zijl. "Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI." Nature medicine 9, no. 8 (2003): 1085-1090.
[2] Kogan, Feliks, Mohammad Haris, Anup Singh, Kejia Cai, Catherine Debrosse, Ravi Prakash Reddy Nanga, Hari Hariharan, and Ravinder Reddy. "Method for high‐resolution imaging of creatine in vivo using chemical exchange saturation transfer." Magnetic resonance in medicine 71, no. 1 (2014): 164-172.
[3] Chan, Kannie WY, Michael T. McMahon, Yoshinori Kato, Guanshu Liu, Jeff WM Bulte, Zaver M. Bhujwalla, Dmitri Artemov, and Peter CM Van Zijl. "Natural D‐glucose as a biodegradable MRI contrast agent for detecting cancer." Magnetic resonance in medicine 68, no. 6 (2012): 1764-1773.
[4] Zhou, Yang, Peter CM van Zijl, Xiang Xu, Jiadi Xu, Yuguo Li, Lin Chen, and Nirbhay N. Yadav. "Magnetic resonance imaging of glycogen using its magnetic coupling with water." Proceedings of the National Academy of Sciences 117, no. 6 (2020): 3144-3149.
[5] Zu, Zhongliang. "Ratiometric NOE (− 1.6) contrast in brain tumors." NMR in Biomedicine 31, no. 12 (2018): e4017.
[6] Huang, Jianpan, Xiongqi Han, Lin Chen, Xiang Xu, Jiadi Xu, and Kannie WY Chan. "Relayed nuclear Overhauser enhancement imaging with magnetization transfer contrast suppression at 3 T." Magnetic resonance in medicine 85, no. 1 (2021): 254-267.
[7] Yadav, Nirbhay N., Xing Yang, Yuguo Li, Wenbo Li, Guanshu Liu, and Peter van Zijl. "Detection of dynamic substrate binding using MRI." Scientific reports 7, no. 1 (2017): 1-7.
[8] Yang, Bo, Xianwen Cao, Chong Wang, Shenghan Wang, and Chenglin Sun. "Investigation of hydrogen bonding in Water/DMSO binary mixtures by Raman spectroscopy." Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 228 (2020): 117704.
[9] Vaisman, Iosif I., and Max L. Berkowitz. "Local structural order and molecular associations in water-DMSO mixtures. Molecular dynamics study." Journal of the American Chemical Society 114, no. 20 (1992): 7889-7896.
[10] McMahon, Michael T., Assaf A. Gilad, Jinyuan Zhou, Phillip Z. Sun, Jeff WM Bulte, and Peter CM Van Zijl. "Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly‐L‐lysine and a starburst dendrimer." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 55, no. 4 (2006): 836-847.
[11] Zaiss, Moritz, Goran Angelovski, Eleni Demetriou, Michael T. McMahon, Xavier Golay, and Klaus Scheffler. "QUESP and QUEST revisited–fast and accurate quantitative CEST experiments." Magnetic resonance in medicine 79, no. 3 (2018): 1708-1721.
[12] Zhou, Yang, Chongxue Bie, Peter CM van Zijl, and Nirbhay N. Yadav. "The relayed NOE mechanism in magnetization transfer and CEST MRI." NMR in Biomedicine.
[13] Zhou, Yang, Chongxue Bie, Peter CM van Zijl, Jiadi Xu, Chao Zou, and Nirbhay N. Yadav. "Detection of electrostatic molecular binding using the water proton signal." Magnetic resonance in medicine (2022).
Figures