2992
Learned spatiotemporal correlation priors for CEST image denoising using incorporated global-spectral convolution neural network1Department of Electronic Science, Xiamen University, Xiamen, China, 2Clinical & Technical Support, Philips Healthcare, Beijing, China
Synopsis
Keywords: CEST & MT, Machine Learning/Artificial Intelligence
Chemical exchange saturation transfer (CEST) MRI is a versatile technique that exploits the saturation transfer between exchangeable protons and water for non-invasive detection of diluted metabolites. Although theoretically promising, the practical application of CEST MRI is still challenged by low CEST contrast and low signal-to-noise ratio (SNR) of acquired images. Here, we proposed a deep learning-based method, dubbed denoising CEST network (DCEST-Net), to fully exploit the spatiotemporal correlation prior embedded in the CEST images and restore noise-free images from their noisy observations. Results suggested that DCEST-Net can achieve better performance compared to the state-of-the-art denoising methods.Introduction
Chemical exchange saturation transfer (CEST) MRI is a versatile technique that exploits the saturation transfer between exchangeable protons and water for non-invasive detection of diluted metabolites(1). Although theoretically promising, the practical application of CEST MRI is still challenged by low CEST contrast and low signal-to-noise ratio (SNR) of acquired images(2). Many efforts have been made to reduce noise and oscillation in the Z-spectrum by exploiting the redundancy information of CEST images, such as PCA(3), MLSVD(4) and NLmCED(5). These methods generally work well, but only limited priors were utilized. In this study, we proposed a deep learning-based method, dubbed denoising CEST network (DCEST-Net), to fully exploit the redundancy embedded in the CEST images and effectively restore noise-free images from their noisy observations. The proposed method was validated under different scenarios in comparison to the state-of-the-art approaches.Method
As illustrated in the schematic in Fig. 1, DCEST-Net consists of two modified U-Nets with different convolution kernel sizes. The pathway with larger convolution kernel size aims to exploit the global redundancy of CEST images, while the pathway with smaller convolution kernel size aims to extract and utilize the prior information along the Z-spectral dimension. The input of the network is masked CEST images with noise, and the output is a denoised CEST image.7-pool Bloch-McConnell equation(6) was used to simulate Z-spectra with different parameters including saturation pulse parameters (saturation power and duration time) as well as concentration, exchange rate, and relaxation rate. The synthetic CEST images were generated by combing Z-spectra with different M0 images. Mean square error (MSE) was used as loss function during the network training with Pytorch 1.12.
$$MSE = \frac{1}{{DHW}}\sum\limits_{D = 0}^{D - 1} {\sum\limits_{H = 0}^{H - 1} {{{\sum\limits_{W = 0}^{W - 1} {\left[ {{X_{{ref}}}\left( {D,H,W} \right) - {X_{denoise}}\left( {D,H,W} \right)} \right]} ^2}}} } $$
The CEST effect was extracted and quantified using the polynomial and Lorentzian line‐shape fitting (PLOF) method as described previously(7). The peak signal-to-noise ratio (PSNR) and structural similarity index (SSIM) of the CEST images and quantitative map obtained by PLOF were calculated.The state-of-the-art CEST denoising methods, NLmCED, MLSVD and BM4D(8), were performed in comparison to the proposed method.
$$PSNR = 10{\log _{10}}\frac{{X_{\max }^2}}{{MSE}}$$
$$SSIM = \frac{{\left( {2{\mu _{{X_{ref}}}}{\mu _{{X_{denoise}}}} + {c_1}} \right)\left( {2{\sigma _{{{X_{ref}}}{{X_{denoise}}}}} + {c_2}} \right)}}{{\left( {\mu _{{X_{ref}}}^2\mu _{{X_{denoise}}}^2 + {c_1}} \right)\left( {\sigma _{{X_{ref}}}^2\sigma _{{X_{denoise}}}^2 + {c_2}} \right)}}$$
Result
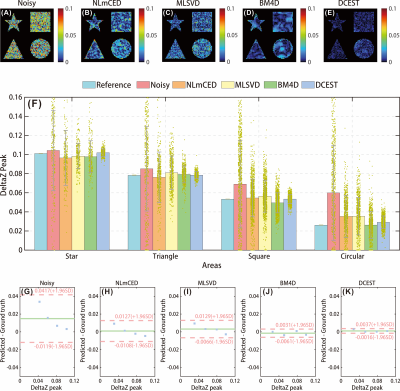
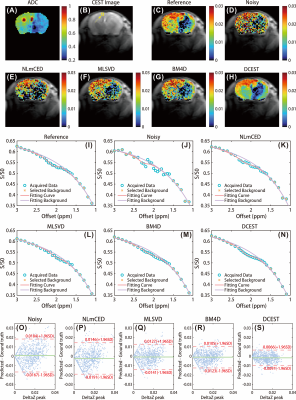
The numerical simulation was performed to validate the efficiency of the proposed method. The ground truth CEST map and representative Z-spectrum are shown in Fig.1. The noisy CEST images were generated by applying 6% Rician noise, and no discernible CEST peak can be observed in the corrupted Z-spectrum, as shown in Fig. 1 (H). We applied the different denoising approaches to the corrupted data, and results indicate that denoised images by DCEST-Net were with significantly improved performance than those by the other approaches (Figures 2B-F), especially for the region with low concentration. From the representative Z-spectra obtained by different methods, DCEST-Net yield the best fidelity compared to the ground truth. In addition, false peaks at 1.5 and 3 ppm were observed in the Z-spectra obtained by NLmCED and BM4D. The difference maps and quantitative analysis of different methods are shown in Fig. 3.Data from in vivo experiments were used to further validate the robustness of DCEST-Net. The reference CrCEST map is shown in Fig. 4(C). The noisy data were obtained by applying 1% Rician noise. As shown in Figures 4C-H, the proposed method can clearly indicate areas of ischemic stroke, which is consistent with the ADC image and reference map. Furthermore, results of DCEST-Net show a narrower error distribution than other approaches as shown in the Bland-Altman plots (Figures 4O-S).
Discussion
In this study, we proposed a deep learning-based technique for CEST image denoising. The efficiency of the proposed method was validated by numerical simulation and in vivo rat experiments.Noise and oscillation reduction in Z-spectrum by exploiting the redundancy information of CEST images has drawn great attention recently due to its wide availability, good compatibility with quantification methods, and no additional data collection required. Methods, including MLSVD, NLmCED, and BM4D, etc, work well generally but still have some shortages. Firstly, the denoising performance of these methods is sensitive to the selection of regularization parameters. Secondly, the matrix decomposition and iterative operations involved in current methods could be time-consuming when comes to data with large sizes or high noise levels. Thirdly, only partial redundancy information was utilized in current denoising models. Deep learning is an emerging and powerful tool and has become the de facto standard for a wide variety of applications. The nonlinear mapping ability, automatic feature extraction, and dimensionality reduction of deep learning are advantageous for CEST image processing. Global and spectral pathways were designed to learn the spatiotemporal correlation prior of CEST images and its superiority was demonstrated in the Result section. Moreover, deep learning-based methods avoid the issues regarding relatively long reconstruction times and empirical selection of the regularization parameters.
Conclusion
DCEST-Net can well exploit the spatiotemporal correlation prior of CEST images for noise reduction, which performed better compared to the state-of-the-art approaches on both numerical simulation and in vivo data.Acknowledgements
This work is supported by Science and Technology Project of Fujian Province, grant number 2022J05013References
1. van Zijl PCM, Lam WW, Xu J, Knutsson L, Stanisz GJ. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage 2018;168:222-241.
2. Zhou J, Zaiss M, Knutsson L, Sun PZ, Ahn SS, Aime S, Bachert P, Blakeley JO, Cai K, Chappell MA, Chen M, Gochberg DF, Goerke S, Heo HY, Jiang S, Jin T, Kim SG, Laterra J, Paech D, Pagel MD, Park JE, Reddy R, Sakata A, Sartoretti-Schefer S, Sherry AD, Smith SA, Stanisz GJ, Sundgren PC, Togao O, Vandsburger M, Wen Z, Wu Y, Zhang Y, Zhu W, Zu Z, van Zijl PCM. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn Reson Med 2022;88(2):546-574.
3. Breitling J, Deshmane A, Goerke S, Korzowski A, Herz K, Ladd ME, Scheffler K, Bachert P, Zaiss M. Adaptive denoising for chemical exchange saturation transfer MR imaging. NMR Biomed 2019;32(11):e4133.
4. Chen L, Cao S, Koehler RC, van Zijl PCM, Xu J. High-sensitivity CEST mapping using a spatiotemporal correlation-enhanced method. Magn Reson Med 2020;84(6):3342-3350.
5. Romdhane F, Villano D, Irrera P, Consolino L, Longo DL. Evaluation of a similarity anisotropic diffusion denoising approach for improving in vivo CEST-MRI tumor pH imaging. Magn Reson Med 2021;85(6):3479-3496.
6. Zhou J, Wilson DA, Sun PZ, Klaus JA, Van Zijl PC. Quantitative description of proton exchange processes between water and endogenous and exogenous agents for WEX, CEST, and APT experiments. Magn Reson Med 2004;51(5):945-952.
7. Chen L, Wei Z, Cai S, Li Y, Liu G, Lu H, Weiss RG, van Zijl PCM, Xu J. High-resolution creatine mapping of mouse brain at 11.7 T using non-steady-state chemical exchange saturation transfer. NMR Biomed 2019;32(11):e4168.
8. Maggioni M, Katkovnik V, Egiazarian K, Foi A. Nonlocal Transform-Domain Filter for Volumetric Data Denoising and Reconstruction. IEEE Transactions on Image Processing 2013;22(1):119-133.
Figures

Figure 1:The proposed network structure for denoising CEST images with 3D convolution. The dimension of the input is 1×D×H×W. The network consists of two modified U-Nets with different convolution kernel sizes. The size of the convolution kernel in the Global pathway is S×S×S, and the size of the convolution kernel in the Spectral pathway is S×1×1. The final output is obtained by a Relu activation function. In this study, S was set to 3, and C was set to 64.
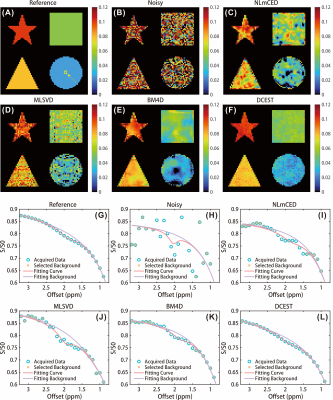
Figure 2:Results of numerical simulation. (A&G) the ground truth CrCEST map and representative Z-spectrum. (B&H) The corrupted CrCEST map and noisy Z-spectrum after applying 6% Rician noise. The denoised CrCEST maps and representative Z-spectrums obtain by NLmCED (C&I), MLSVD (D&J), BM4D (E&K), and DCEST-Net (F&L).