2991
In silico optimization of iopamidol CEST MRI for renal pH mapping at 3 Tesla1F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 2The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 4Institute of Neuroradiology, Friedrich-Alexander University Erlangen-Nürnberg (FAU), Erlangen, Germany, 5International Center for Spinal Cord Injury, Kennedy Krieger Institute, Baltimore, MD, United States, 6Physical Medicine and Rehabilitation, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Keywords: CEST & MT, CEST & MT
In vivo optimization of CEST iopamidol contrast in human subjects is complicated and requires multiple examinations and injections of the agent. To address this challenge, we propose application of a numerical approach that utilizes exchange rates determined under physiological conditions at 17.6T to perform kidney-like multi-pool Bloch-McConnell simulations for in silico optimization of saturation parameters for 3T applications. Our results suggest that the iopamidol-based CEST MRI is sensitive to pH in the range between 6 and 7.2 with the optimal results when short CEST saturation pulses (3x100 ms), low B1 strength (B1~0.8 µT) and short recovery time (Trec~T1w) are applied.Introduction
As the kidneys play a predominant role in maintaining internal acid-base homeostasis, pH may be a useful biomarker of renal function. With its high sensitivity to pH, iopamidol-enhanced chemical exchange saturation transfer (CEST) MRI is potentially an attractive tool for the in vivo detection of kidney disease. Despite encouraging results from several preclinical studies1–3, the feasibility of CEST-based renal pH mapping at clinical field strengths has not been fully determined. A main reason is the complexity of CEST contrast optimization in human subjects, which typically requires multiple MRI scanning sessions and injections of contrast agent. The objective of this study was to address this limitation by applying a recently proposed numerical approach4. First, exchange rates of iopamidol protons at physiological conditions were quantified and used to create a kidney-like system with realistic water relaxation rates at 3T and a semi-solid magnetization transfer (ssMT) component. In the next step, this numerical multi-pool model was employed in Bloch-McConnell simulations to investigate changes in CEST signal and saturation transfer ratio depending on the presaturation parameters allowing to find the optimal parameters for renal pH mapping on 3T scanners.Methods
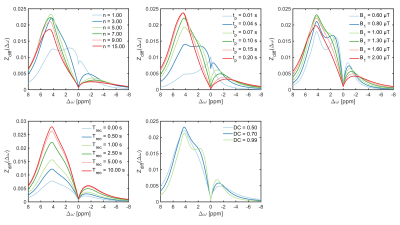
Six solutions of 25 mM iopamidol dissolved in phosphate-buffered saline at different pH values between 5.8 and 7.4 were prepared and examined on a 17.6 T spectrometer. The temperature of the samples was kept constant at 37°C. For CEST measurements, a rectangular pulse of 7.2 s at five different saturation field strengths (B1) between 2.4 and 4.8 µT was used. Z-spectra were sampled at 201 frequency offsets between ±10 ppm.Data analysis, simulations und Bloch-McConnell fitting were performed in Matlab5. The simulation files are available on github (https://github.com/cest‐sources/BM_sim_fit). All 30 Z-spectra were fitted simultaneously using a multi-pH-multi-B1 method by extending the Bloch equations to use pH as a parameter, and assuming that the change in exchange rate can be expressed as a sum of changes in base- and water-catalyzed exchange (kx=kx1·10pH-7+kx0), as the buffer contribution remains constant. Subsequently, using the estimated exchange rates and kidney tissue properties at 3T6,7, a kidney-like model including a water pool, ssMT pool and four iopamidol proton pools (see Figure 1 for assignment) was created to optimize the saturation parameters. Iopamidol contrast (Zdiff) was calculated by subtracting the Z-spectra with (Zpost:6 pool) from those without (Zpre:2 pool) the iopamidol pools activated. In the next step, the saturation transfer ratio STratio=Zdiff (4.3 ppm)/Zdiff (5.5 ppm) was determined for different combinations of B1 and number of saturation pulses (n) of length (tp) of 0.1 s (i.e varying the total saturation time).
Results
The results of the multi-pH-multi-B1 fitting at 17.6 T are displayed in Figure 1, which reveals that the exchange rates of amide protons at 5.5 and 4.3 ppm increased from 106±9 s-1 to 3796±83 s-1 and 34±4 s-1 to 1199±37 s-1, respectively, in the pH range from 5.8 to 7.4.Figure 2 shows exemplary results of the CEST parameter optimization process with respect to iopamidol contrast at an average pH of 6.78. At this pH value, the optimal parameters yielding ~2.5% CEST effect at 4.3 ppm and ~1.5% at 5.5 ppm were: n=3, tp=0.1 s, B1=1.0 µT, Trec=2.5 s and DC=0.5.
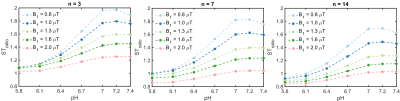
The ratiometric CEST signals calculated for different combinations of n and B1 values are displayed in Figure 3. These results suggest that the ratiometric CEST analysis allows measurements of pH in the range between 6 and 7.0 with the highest sensitivity obtained for n=3 and B1=0.8 µT.
Discussion
In this study, we determined iopamidol exchange rates under physiological conditions at 17.6T, and used these to create a kidney-like system including realistic water relaxation times and ssMT component at 3T. This model was then employed in Bloch McConnell simulations with different saturation parameters to establish optimal saturation conditions for in vivo renal pH mapping at 3T.The exchange rates estimated here are slightly higher than those obtained at room temperature in a previous study9, which is consistent with faster exchange at higher temperatures. Still, with highest exchange rate of about 4000 s-1 at pH 7.4 and relatively large chemicals shifts of the amide groups at 4.3 and 5.5 ppm, iopamidol appears to be an attractive CEST agent for clinical applications at 3T. The optimal CEST parameters found in our study suggest that sufficient saturation of both amide groups and high sensitivity to pH in the physiological range can be achieved using short RF pulses (3x100 ms) with low B1~0.8 µT, and short Trec=2.5 s. This combination is particularly favorable for contrast-enhanced CEST acquisitions, which are limited by rapid renal clearance of iopamidol that determines temporal resolution.
The numerical approach applied in our study can be used as guidance for in vivo optimization in human subjects without the need for multiple injections of contrast agent during a single examination. Nevertheless, the optimal parameters predicted by our simulations should be verified experimentally.
Conclusion
Using iopamidol exchange rates determined in the physiological pH range and temperature, the sensitivity of pH imaging was found to be optimal when short saturation time and a relatively low B1 was applied. The numerical approach used here can aid the optimization of iopamidol CEST MRI without the need for injections.Acknowledgements
This work is supported by NIH grant 5R01DK121847-02 and by a Kennedy Krieger Institute Goldstein Innovation and Collaboration Award.References
1. Longo DL, Busato A, Lanzardo S, Antico F, Aime S. Imaging the pH evolution of an acute kidney injury model by means of iopamidol, a MRI-CEST pH-responsive contrast agent. Magnetic Resonance in Medicine. 2013;70(3):859-864
2. Longo DL, Cutrin JC, Michelotti F, Irrera P, Aime S. Noninvasive evaluation of renal pH homeostasis after ischemia reperfusion injury by CEST-MRI. NMR in Biomedicine. 2017;30(7):e3720.
3. Stabinska J, Singh A, Haney NM, et al. Noninvasive assessment of renal dynamics and pH in a unilateral ureter obstruction model using DCE MR-CEST urography. Magnetic Resonance in Medicine. n/a(n/a).
4. Zaiss M, Anemone A, Goerke S, et al. Quantification of hydroxyl exchange of D-Glucose at physiological conditions for optimization of glucoCEST MRI at 3, 7 and 9.4 Tesla. NMR in Biomedicine. 2019;32(9):e4113.
5. Zaiss M, Angelovski G, Demetriou E, McMahon MT, Golay X, Scheffler K. QUESP and QUEST revisited – fast and accurate quantitative CEST experiments. Magnetic Resonance in Medicine. 2018;79(3):1708-1721.
6. Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic Resonance in Medicine. 2005;54(3):507-512.
7. Jiang K, Ferguson CM, Grimm RC, Zhu X, Glockner JF, Lerman LO. Reliable Assessment of Swine Renal Fibrosis Using Quantitative Magnetization Transfer Imaging. Investigative Radiology. 2022;57(5):334-342.
8. Brenner BM, Levine, Samuel A. Brenner and Rector’s the Kidney. Vol 2. 8th ed. Saunders Elsevier; 2008.
9. Sun PZ, Longo DL, Hu W, Xiao G, Wu R. Quantification of iopamidol multi-site chemical exchange properties for ratiometric chemical exchange saturation transfer (CEST) imaging of pH. Phys Med Biol. 2014;59(16):4493-4504.
Figures