2990
Imaging Extracellular Lactate with Lanthanide-PCTA-based PARACEST Agents
Remy Chiaffarelli1,2, Paul Jurek3, Pedro Cruz4, Max Zimmermann1,2, Carlos Geraldes4, Garry Kiefer3, and André Ferreira Martins1,2
1Werner Siemens Imaging Center, Department of Preclinical Imaging and Radiopharmacy, University Hospital Tuebingen, Tuebingen, Germany, 2Cluster of Excellence iFIT (EXC 2180) “Image-Guided and Functionally Instructed Tumor Therapies”, University of Tuebingen, Tuebingen, Germany, 3Macrocyclics, Inc., Plano, TX, United States, 4Coimbra Chemistry Center - Institute of Molecular Sciences (CQC-IMS), University of Coimbra, Coimbra, Portugal
1Werner Siemens Imaging Center, Department of Preclinical Imaging and Radiopharmacy, University Hospital Tuebingen, Tuebingen, Germany, 2Cluster of Excellence iFIT (EXC 2180) “Image-Guided and Functionally Instructed Tumor Therapies”, University of Tuebingen, Tuebingen, Germany, 3Macrocyclics, Inc., Plano, TX, United States, 4Coimbra Chemistry Center - Institute of Molecular Sciences (CQC-IMS), University of Coimbra, Coimbra, Portugal
Synopsis
Keywords: CEST & MT, CEST & MT, PARACEST, Metabolism
Lactate accumulation in the tumor microenvironment is a hallmark of cancer aggressiveness. Here, we report a method to image extracellular lactate using shiftCEST MRI and Yb- and EuPCTA Shift Reagents (SRs). In the presence of lactate, the SRs shift the lactate OH-CEST signal away from the water signal by ~14 ppm (EuPCTA) and ~95 ppm (YbPCTA), allowing for quantitative detection of extracellular lactate produced by cancer cells. In vivo studies confirmed the detection and fast renal elimination of the lactate*YbPCTA into the bladder by shiftCEST MRI. Overall, these results provide new insights into developing innovative non-invasive metabolic MRI.Introduction
Cancer cells overproduce lactate even in the presence of high oxygen and glucose levels, a phenomenon called Warburg effect. Most of the lactate produced by cancer cells builds up in the extracellular environment, affecting the tumor and the homeostasis of the surrounding tissues. Hence, a method for imaging extracellular lactate produced by the tumors is of utmost importance to better understand tumor metabolism. Previous reports showed that extracellular lactate could be detected using a paraCEST shift reagent (SR), EuDO3A, that binds lactate and shifts its OH-CEST signal away from the water signal1. We report here the first results of a new generation of PCTA-based SRs, Eu- and YbPCTA (q = 2 complex), able to image extracellular lactate by CEST MRI with improved kinetic inertness.2Methods
Phantoms containing 20 mM Eu- and YbPCTA dissolved in water or serum in the presence/absence of lactate (5-600 mM) were imaged in a 7 T preclinical MR scanner (Bruker Biospec 70/30) using an established CEST FISP sequence and different sets of 5 seconds, rectangular shaped, continuous pre-saturation pulses (B1 ranging from 2 to 21 μT), to generate CEST spectra, lactate-SRs calibration curves, and to determine binding affinities. Exchange rates were calculated using the Omega plot method. Relaxivity r1 and r2 values of the SRs (0-10 mM) were calculated from T1 and T2 maps acquired at 7 T at room temperature and 37°C. Structural chemical analysis of 1:1 50 mM lactate-SR complexes was performed with high-resolution 1H and 13C NMR recorded on a Bruker AVANCE III 400 NMR (9.4 T) spectrometer at different pH and temperatures. Phantoms containing cell culture supernatants and SRs were acquired with the same protocols to detect lactate excreted by MC-38 cancer cells over 48h in standard culture conditions. Lactate concentrations calculated from CEST images were cross-validated with an enzymatic assay. In vivo CEST detection and SR excretion was tested in healthy mice. After i.v. injection of 0.5 mmol/Kg of lactate*YbPCTA, CEST images at 109 ppm were acquired every 5 minutes using 3 seconds, 14 μT saturation pulses, then the dynamics of MR signal were determined for bladder and muscle tissue.Results and Discussion
Eu- and YbPCTA complexes are able to shift the lactate –OH CEST signal far away from its typical chemical shift (0.6-0.8 ppm) and the water protons (0 ppm), resulting in a CEST effect easily detectable by CEST MRI (Fig. 1B). The lactate-YbPCTA complex displays a larger chemical shift (> 95 ppm) compared to EuPCTA (10-18 ppm). Exchange rates (kex) presented a minimum at neutral pH for EuPCTA and pH 6 for YbPCTA, resulting in a complementary CEST effect for imaging lactate at different pHs (Fig.1, table 1). r1 relaxivity, a key governing parameter for CEST detection, was determined in serum at 37°C to be 0.009 mM-1∙s-1 for YbPCTA and 0.003 mM-1∙s-1 for EuPCTA. Contrary to previous reports for LnDO3A complexes (3:1 SAP:TSAP ratio of structural diastereomers), high-resolution 1H NMR at various temperatures detected only the presence of a TSAP diastereomer in solution for the lactate*Yb- and Eu-PCTA ternary complexes. Titrations of lactate showed a perfect linear correlation between lactate concentration and CEST signals produced by Eu- and Yb- complexes at neutral (Fig.1C) and acidic pH. We performed lactate titrations to determine the binding affinity of lactate to Eu- and YbPCTA. The obtained results were similar to the previously reported SR (EuDO3A)1 (Fig.1, table 1). The potential of the SRs was demonstrated by quantifying the extracellular lactate produced by MC-38 cells growing in cell culture using CEST images. The chemical shifts produced by Eu- and YbPCTA-lactate are large enough to exclude any possible interference from the water and other endogenous CEST signals. LDH assays confirmed the high accuracy of extracellular lactate detection by the Ln-PCTA complexes. Selectivity of detection in the presence of potentially confounding OH- and NH- resonances was further tested by CEST MRI using serum, fresh cell culture medium, citrate, bicarbonate, and phosphate. In vivo studies with healthy mice also confirmed the efficient detection of extracellular lactate in the muscle and bladder by shiftCEST upon i.v. injection of 0.5 mmol/Kg lactate*YbPCTA. The ternary complex lactate*YbPCTA is cleared fast into the bladder suggesting a renal elimination, with almost total clearance at 60 min post-injection (Fig. 2A, 2B).Conclusions
We demonstrated for the first time that alternative lanthanide-PCTA complexes could successfully detect extracellular lactate by CEST MR imaging in vitro and in vivo. The superior kinetic inertness and thermodynamic stability of the Ln-PCTA complexes make them optimal candidates for safe translational imaging studies. These results provide the framework to develop and characterize new SRs to image extracellular lactate produced in tumors and metabolic compartmentalization by non-invasive CEST MRI.Acknowledgements
We thank Dr. Sabrina Hoffmann (University Hospital of Tuebingen) for the technical and organizational help; Prof. Mark D. Pagel (MD Anderson Cancer Center) for the help in the implementation of CEST MR sequences and the useful discussions; Debra L. Cranmer (Macrocyclics, Inc.) for the organizational support.References
[1] Zhang, L., et al., Imaging Extracellular Lactate In Vitro and In Vivo Using CEST MRI and a Paramagnetic Shift Reagent. Chemistry, 2017. 23(8): p. 1752-1756.
[2] Rojas-Quijano, F.A., et al., Lanthanide(III) complexes of tris(amide) PCTA derivatives as potential bimodal magnetic resonance and optical imaging agents. Chemistry, 2009. 15(47): p. 13188-200.
Figures
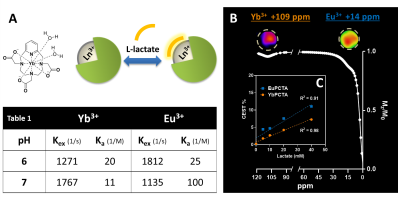
(A)
YbPCTA structure and schematic of lactate-SRs binding. (B, top) CEST images
of phantoms containing 1:1 20 mM solutions of SRs and lactate at pH 7, acquired
using 5 seconds, 16 µT saturation pulses. (B, bottom) Z-spectrum showing
CEST signal of 20 mM lactate-SR complexes at 109 ppm (Yb), 14 ppm (Eu), fitted
to Lorentzian shapes based on a two-pool model (water, lactate). (C)
Plot of CEST effect at 109 ppm (Yb) or 14 ppm (Eu) versus lactate
concentration, pH 7. Dotted lines: linear regression.

(A)
MR images of a mouse injected i.v. with 1:1 0.5 mmol/Kg lactate-YbPCTA. Left,
TurboRARE T2-weighted image; right, overlay of anatomy and CEST
images acquired at 109 ppm before (Pre-Inj.) and 60 minutes after injection,
using 3 seconds, 14 µT saturation pulses. (B) Dynamic changes of the
CEST MR signal determined as [1-(Mz(t)/Mz(pre-injection))]
% in muscle and bladder.
DOI: https://doi.org/10.58530/2023/2990