2987
Metabolic Imaging of malignant gliomas during immunotherapeutic intervention using chemical exchange saturation transfer (CEST) MRI at 9.4T1Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 2Clinical Cooperation Unit Neurooncology, German Cancer Consortium (DTK) within the German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Clinical Cooperation Unit Neuroimmunology and Brain Tumor Immunology, German Cancer Consortium (DTK) within the German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Department of Neurology, University Medical Center Mannheim, Heidelberg University, Mannheim, Germany, 5Department of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 6Department of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 7Clinic for Neuroradiology, University Hospital Bonn, Bonn, Germany, 8School of Biomedical Engineering, Science and Health Systems, Drexel University, Philadelphia, PA, United States, 9Department of Neurology, Heidelberg University Hospital, Heidelberg, Germany
Synopsis
Keywords: CEST & MT, High-Field MRI, Glioma
CDNP-R848, an experimental immunotherapeutic TLR7/8 agonist, showed high treatment efficacy with a significant tumor volume reduction and led to a re-normalization of the metabolic properties (MTRex Amide, MTRex Amine and MTRex NOE) of the tumor in relation to the healthy brain, with distinct differences to vehicle treatment. The clinical relevance of CEST imaging appears to be localizing active tumor areas and a better understanding of tumor heterogeneity. In the future, we aim to better characterize the origin of the CEST contrast by correlated histological and spatial metabolic analysis.
Background
MRI is the main modality for the initial diagnosis of primary brain tumors and for differentiating them from brain metastases or other intracranial lesions. However, differentiation between tumor progression and pseudoprogression, as well as dissecting metabolic information, remains challenging as sensitivity and specificity of current advanced imaging techniques are limited.1 Here we hypothesized that chemical exchange saturation transfer (CEST), a non-invasive imaging technique based on magnetization transfer from protons in biomolecules to water2, can detect metabolic changes that occur during immunotherapy of glioma.Methods
CEST imaging was implemented at a 9.4 T Bruker small animal MRI scanner. First, sequence stability was tested in a test-retest design in healthy male C57BL/6 mice (n=6 mice). To assess CEST imaging in glioma, we used the GL261 glioma model. Gl261 cells were orthotopically implanted into the right striatum of 17 female C57BL/6 mice. Eight mice were treated with the experimental immunotherapeutic TLR7/8 agonist CDNP-R8483 (14, 17 and 20 days post-implantation), while the remaining mice (n=9) received CDNP vehicle control. Longitudinal MRI was performed before (week 2 after implantation), during (week 3) and after therapy (week 4) and included T2w and CEST. Images were evaluated by segmentation of the tumor and normalization with the contralateral, healthy brain parenchyma.Results
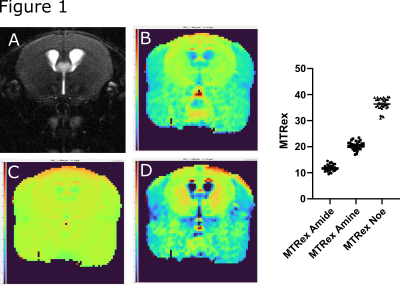
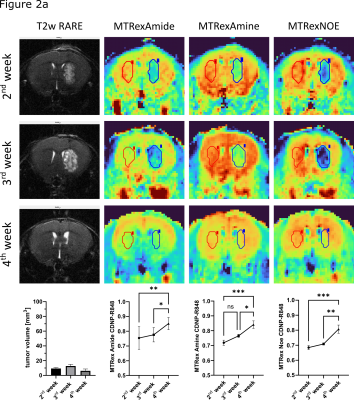
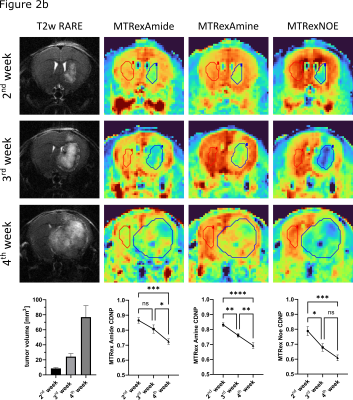
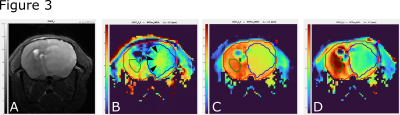
Test-retest reliability showed a good stability of the sequence with comparable values in all three MTRex signals (Amide, Amine, NOE) at three different time points and in different mice (n=6) (Fig.1). In the Gl261 glioma model, CDNP-R848 led to partial response that was characterized by a re-normalization and significant increase in the MTRex signaling ratio (tumor/healthy brain) over the treatment course (comparison 2nd week vs. 4th week: MTRex Amide: p=0.0071; MTRex Amine: p=0.0005; MTRex NOE: p=0.0004), while CDNP vehicle treated mice showed progressive disease and a consecutive CEST ratio drop over time (comparison 2nd week vs. 4th week: MTRex Amide: p<0.0001; MTRex Amine: p=0.0003; MTRex NOE: p=0.0002; Fig.2a/b). There was pronounced tumor heterogeneity in the MTRex signals, notably in the MTRex-amide signal in the late tumor stage of control mice, whereas the T2w-RARE signal was predominantly homogeneous (week 3, Fig.3).Discussion
CDNP-R848 showed high treatment efficacy and led to a re-normalization of the metabolic properties of the tumor in relation to the healthy brain, with distinct differences to vehicle treatment. The clinical relevance of CEST imaging appears to be localizing active tumor areas and a better understanding of tumor heterogeneity. In the future, we plan to better characterize the origin of the CEST contrast by including both histological and spatial metabolic analyses.Conclusion
CEST imaging is a promising approach to analyze molecular and metabolic heterogeneity in glioma models and, ultimately, patients.Acknowledgements
No acknowledgement found.References
1. Young, R. J. et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 76, 1918-1924, doi:10.1212/WNL.0b013e31821d74e7 (2011).
2. Mamoune, K. E., Barantin, L., Adriaensen, H. & Tillet, Y. Application of Chemical Exchange Saturation Transfer (CEST) in neuroimaging. J Chem Neuroanat 114, 101944, doi:10.1016/j.jchemneu.2021.101944 (2021).
3. Rodell, C. B. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng 2, 578-588, doi:10.1038/s41551-018-0236-8 (2018).
Figures