2978
Test-retest repeatability of motility MRI in healthy volunteers and patients with Crohn’s disease1Department of Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Takeda Pharmaceuticals, Cambridge, MA, United States, 4Motilent Ltd, London, United Kingdom, 5Department of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Keywords: Digestive, Quantitative Imaging, bowel, Crohn's disease
Our study aims to determine test-retest repeatability in motility (bowel wall motion) index derived from CINE-MRI measured in involved and uninvolved small bowel in patients with Crohn’s disease and in healthy volunteers, to determine a clinically significant threshold of motility change in response to treatment.Motility was lower in the involved areas compared to uninvolved small bowel, and in the uninvolved small bowel ofpatients compared to that of healthy volunteers. We found moderate test-retest repeatability in the motility index of involved bowel (provide average CV) and in the ratio ofmotility indices in involved small bowel and in uninvolved small bowel.Introduction
Patients with Crohn’s disease (CD) often suffer from dysmotility of the small bowel1, 2, in particular in the area of involvement. Growing interest in motility MRI and development of associated FDA-cleared image processing technology has led to the ability to monitor small bowel motility noninvasively and with precision 1-4. It would be important to determine the degree of dysmotility in an affected region of the ileum and to quantify that degree of “disease” compared to unaffected (normal appearing) ileum in the same patient, so that the patient can be his/her own control in studies of therapeutic efficacy. Herein we aim to determine the test-retest repeatability of differences in motility between involved bowel and unaffected small bowel towards generating a threshold at which this difference meets clinical significance.Methods
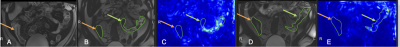
In a single-center, IRB-approved, ongoing prospective study, 6 patients (M/F:1/5, age 38±10 (28-49) years) with endoscopy and pathology-confirmed CD involving the ileum and starting a new biologic therapy, and 4 healthy volunteers (M/F:2/2, age 29±5 (28-34) years) with no history of inflammatory bowel disease, were evaluated by CINE-MRI on 1.5T Siemens Magnetom Sola fit or Aera scanners. The clinical MR enterography (MRE) was conducted before the start of new biologic therapy in CD patients. All subjects including volunteers were scanned in supine position, instructed to fast for 4 h and given 1000 ml of a mannitol and sorbitol oral contrast preparation (Breeza, Beekly Medical) to drink over 40 minutes before the MRE. MRE consisted of routine sequences acquired without and with gadolinium contrast agent (Gadavist, Bayer; IV contrast was not given to volunteers) and research sequences. CINE-MRI was acquired (TR/TE/FA: 473 ms/1.32 ms/59°, FOV 390x384 mm2, matrix size 228x224, 8 mm) with 20 dynamics over a breath-hold of 17s. Five 8 mm thick coronal slices of the affected ileum were acquired in a coffee-break test/retest fashion. The affected small bowel area was determined according to evaluation of the previously acquired T2-weighted axial and coronal anatomical scans, by an abdominal radiologist present for the duration of the scan. For the test-retest experiment, subjects were asked to leave the scanner and return after a 3-minute sitting break, after which they were repositioned and a new motility series was acquired. The motility index was calculated in proprietary software (GIQuant, Motilent, London, UK) from the CINE images as the standard deviation of the determinant of the Jacobian matrix for the deformation vector field in each pixel3. One abdominal radiologist independently placed one ROI on the area of involvement (where visible) and one ROI in distal healthy ileumin each of the five slices (Fig. 1). The area of involvement was determined based on bowel wall enhancement on T1w contrast-enhanced images. For each subject, the average of the motility index in the area of involvement and unaffected ileum, weighted according to ROI size, was calculated. The ratio of motility indices in the area of involvement and unaffected bowel was calculated for patients. Test/retest repeatability of motility index for the area of involvement and unaffected bowel, as well as for the ratio, was assessed by coefficient of variation (CV, %). The motility index was compared between involved and unaffected bowel, and in unaffected bowel between patients and volunteers by Mann-Whitney tests.Results
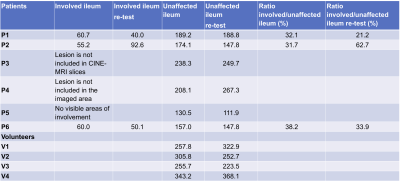
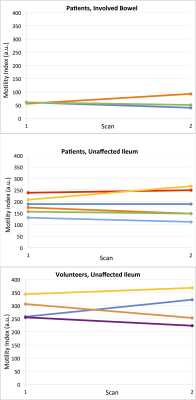
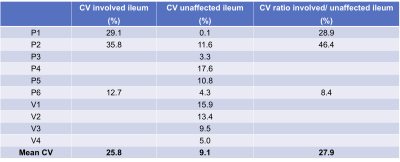
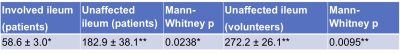
The area of involvement was visible on the CINE-MRI test and re-test images in3 out of the 6 patients (Figure 2,Table 1). In one patient (Table 1, P5), there was no radiologically visible area of involvement, despite the presence of aphthous ulcers in the terminal ileum on endoscopy, and biopsy-confirmed CD; of note, motility index values in the normal appearing ileum are lower for this patient than for the others (Figure 2). In the patients, motility in the involved bowel was 34% of the motility values in unaffected bowel (Table 1; Mann-Whitney p=0.0238). The mean test-retest CV of the motility index in the area of involvement was 25.8%, 8% for unaffected ileum, and 27.9% for the ratio of motility indices in the area of involvement and unaffected ileum in the patients, and 10.9% for ileum in volunteers (Table 2).Motility in the unaffected ileum of the patients was significantly smaller than the ileum motility in volunteers (Table 3; p=0.0095).
Discussion
Test-retest repeatability of the motility index in healthy subjects was comparable to published findings3,5. Our initial results also confirm previous findings of hypomotility in the uninvolved small bowel of patients with CD compared to healthy volunteers1,6, which may be due to global inflammatory changes in radiologically unaffected ileum and will be a topic of future research. Our study is the first to measure test-retest repeatability of the motility index in the involved bowel in patients with CD. While moderate, test-retest repeatability of motility of the involved bowel and the ratio of involved to unaffected bowel could help establish a clinical threshold of response to treatment of >50% (2xCV) increase in motility or motility ratio. Changes in motility of 15%-75% have been previously observed in response to biological treatment2,4.Conclusion
Knowledge of test-retest repeatability of the motility index can help establish a clinically meaningful threshold in hypomotility improvement in response to treatment of patients with CD.Acknowledgements
This work was sponsored by a collaborative grant from Takeda Pharmaceuticals.References
1. Åkerman A, Månsson S, Fork FT, et al. Computational postprocessing quantification of small bowel motility using magnetic resonance images in clinical practice: An initial experience. J Magn Reson Imaging. Aug 2016;44(2):277-287.
2. Dillman JR, Tkach JA, Imbus R, Towbin AJ, Denson LA. MRI-Based Characterization of Intestinal Motility in Children and Young Adults With Newly Diagnosed Ileal Crohn Disease Treated by Biologic Therapy: A Controlled Prospective Study. AJR Am J Roentgenol. Oct 2022;219(4):655-664.
3. Menys A, Taylor SA, Emmanuel A, et al. Global small bowel motility: assessment with dynamic MR imaging. Radiology. Nov 2013;269(2):443-450.
4. Plumb AA, Menys A, Russo E, et al. Magnetic resonance imaging-quantified small bowel motility is a sensitive marker of response to medical therapy in Crohn's disease. Aliment Pharmacol Ther. Aug 2015;42(3):343-355.
5. Menys A, Plumb A, Atkinson D, Taylor SA. The challenge of segmental small bowel motility quantitation using MR enterography. Br J Radiol. Aug 2014;87(1040):20140330.
6. Khalaf A, Hoad CL, Menys A, et al. Gastrointestinal peptides and small-bowel hypomotility are possible causes for fasting and postprandial symptoms in active Crohn's disease. Am J Clin Nutr. Jan 1 2020;111(1):131-140.
Figures