2976
Predict intraoperative hemorrhage during curettage treatment of cesarean scar pregnancy using free-breathing GRASP DCE-MRI1Radiology, Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Scienc, Chengdu, China, 2Gynecology, Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Scienc, Chengdu, China, 3MR Scientific Marketing, Siemens Healthineers, Shanghai, China
Synopsis
Keywords: Urogenital, Reproductive, Cesarean Scar Pregnancy; Dynamic Magnetic Resonance Imaging; Dynamic Contrast Enhancemen; Prediction Ability; Surgical Risk
Cesarean scar pregnancy (CSP) is a rare form of ectopic pregnancy with curettage often being the first-line treatment. However, uncontrollable intraoperative hemorrhage is one of common complications during the treatment, making its prediction a critical for treatment planning. The aim of this study was to explore the feasibility of using GRASP DCE-MRI to characterize peritrophoblastic perfusion and predict intraoperative hemorrhage for CSP. The study found that presurgical Wash-in, TTP, iAUC, Ktrans, and Ve were significantly different between hemorrhage and non-hemorrhage groups identified during curettage. The ROC-AUC analysis further demonstrated their capability of serving as intraoperative hemorrhage predictors for CSP.Introduction
Cesarean scar pregnancy (CSP) is a rare form of ectopic pregnancy with potential severe complications such as uterine rupture and massive bleeding, making it life-threatening1. Therefore, once diagnosed, immediate termination of pregnancy by surgical interventions including suction curettage is often taken as the first-line treatment2. However, uncontrollable intraoperative hemorrhage is not rare during the treatment, which could results in hysterectomy and loss of future fertility, rendering presurgical assessment of massive hemorrhage inevitable3. Peritrophoblastic perfusion has been identified as one of risk factors among others including gestational age, size and myometrial layer thickness3-4. Peritrophoblastic perfusion in CSP is currently often assessed by ultrasound5, while the potential of MRI to serve as an alternative has not been fully explored. Although dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has been used to locate gestational sac and assess neovascular perfusion4, conventional DCE-MRI is known to be sensitive to motion and requires breath-holding during scan, resulting in poor temporal and spatial resolution. To overcome this limitation, a novel DCE-MRI technique known as Golden-angle RAdial Sparse Parallel (GRASP) imaging was invented to achieve an acquisition with much higher temporal resolution without breath-holding5, thus increasing patient comfort while allowing higher spatial resolution images to be acquired and more accurate perfusion to be quantified. The aim of this study was to explore the feasibility of using GRASP DCE-MRI to characterize peritrophoblastic perfusion and evaluate its prediction power for intraoperative hemorrhage during curettage of CSP.Method
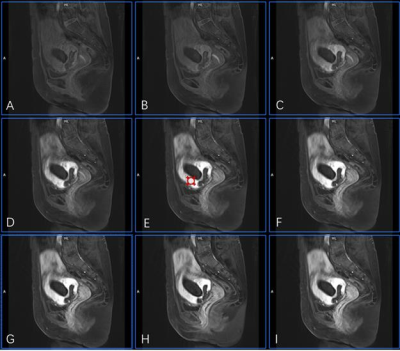
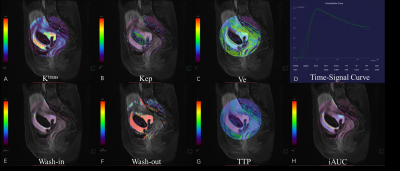
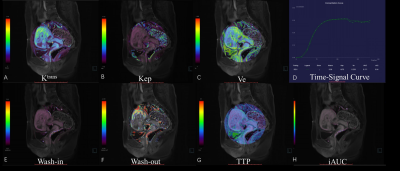
Between January 2021 and July 2022, 49 patients with CSP (age range: 25-48 years, mean: 33.6±3.9 years; menopause time: 42-90 days, mean:55.41±11.75 days; interval between current and last cesarean section: 1-8 years, mean: 4.06±1.62 years; cases of virginal bleeding: 47/49), who underwent curettage without any previous interventions, were retrospectively selected for this study. Three days prior to curettage, both anatomical MRI and GRASP DCE-MRI were performed for each patient at a 3.0 T MR scanner (MAGNETOM VIDA, Siemens Healthcare, Erlangen, Germany) with an 18-channel phased array surface coil. During DCE-MRI, Gd-DTPA was intravenously injected at a dose of 0.1 mmol/kg and a flow rate of 3 ml/s, and after a 15-second delay GRASP acquisition was followed with following parameters: matrix=320×320, FOV = 256 mm × 256 mm, slice thickness = 4 mm, TR = 4.09 ms, TE = 2.04 ms, temporal resolution = 10.4 s and phases = 25. The images acquired was analyzed by Tissue 4D (Siemens Healthcare, Erlangen, Germany). First, images from the phase with strongest contrast-enhancing were chosen and region of interests (ROIs) were defined on enhanced gestational sac villi around previous incision scar (Figure 1). This was done independently by two radiologists who were blinded to the degree of hemorrhage during curettage. Then, pharmacokinetic parameters including Wash-in, Wash-out, TTP, iACU, Ktrans, Kep, and Ve were calculated within the ROIs and averaged between the two raters. The amount of intraoperative bleeding was recorded by a gynecologist who performed curettage and used to divide patients into two groups: non-hemorrhage (blood loss<=200 mL) and hemorrhage (blood loss>200 mL)6. The measured pharmacokinetic parameters from the two groups were statistically compared using the t-test or Mann-Whitney U test with a significant level set to be p < 0.05. The receiver operating characteristic (ROC) curve was constructed and the area under the curve (AUC) was calculated to evaluate each parameter’s capability in intraoperative hemorrhage subgroup classification. All analyses were done using SPSS 22.0.Results
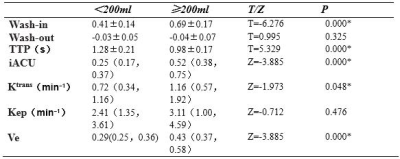
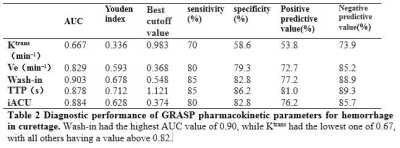
Out of 49 patients, 20 of them had intraoperative hemorrhage (blood loss>200 mL) during curettage while 29 of them did not(Figure 2, 3). The hemorrhage group had larger Wash-in, iAUC, Ktrans , Ve and shorter TTP than the non-hemorrhage group, and their differences were statistically significant (Table 1). From the the ROC curves of these parameters, the Wash-in had the highest AUC value of 0.90, while Ktrans had the lowest one of 0.67, with all others having a value above 0.82 (Table 2). The other two parameters Wash-out and Kep were not significantly different between the two groups.Discussion
To our knowledge, no previous study investigated the feasibility of GRASP DCE-MRI to predict intraoperative hemorrhage during surgical treatment of CSP. It is known that during placentation extravillous trophoblasts modify uterine vessels to promote placental blood flow and cytotrophoblasts give rise to placental villi that undergoes vasculogenesis and angiogenesis7. This in turn leads to peritrophoblastic hyperperfusion but at a variable degree in patients with CSP, which was likely demonstrated by the perfusion quantified by GRASP DCE-MRI in our study. According to their pharmacokinetic property, the larger Wash in, iAUC and shorter TTP reflect increased blood supply while the larger Ktrans and Ve reflect increased vessel permeability, and this probably explains why patients with these kinds of perfusion could be reliably assigned to the hemorrhage group. With this perfusion quantification capability, plus its excellent anatomical contrast for gestational sac and myometrial layer, MRI is anticipated to be a better comprehensive intraoperative hemorrhage risk assessment tool for CSP than ultrasound.Conclusion
GRASP DCE-MRI can predict intraoperative hemorrhage during curettage treatment of CSP, thus aid the choice of personalized treatment. Future study is needed to compare its effectiveness with that of other risk factors from both anatomical MRI and ultrasound.Acknowledgements
No acknowledgement found.References
1. Petersen KB, Hoffman E, Larsen CR, et al. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016 ;105(4):958-67.
2. Le A, Li M, Xu Y, et al. Different Surgical Approaches to 313 Cesarean Scar Pregnancies. J Minim Invasive Gynecol. 2019;26(1):148-152.
3. Wang Q, Ma H, Peng H, et al. Risk factors for intra-operative haemorrhage and bleeding risk scoring system for caesarean scar pregnancy: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2015;195:141–5.
4. Gui T, Peng P, Liu X, et al. Clinical and ultrasound parameters in prediction of excessive hemorrhage during management of cesarean scar pregnancy. Therapeutics and Clinical Risk Management. 2017;13:807–812.
5. Wu R, Klein MA, Mahboob S, et al. Magnetic resonance imaging as an adjunct to ultrasound in evaluating cesarean scar ectopic pregnancy. J Clin Imaging Sci. 2013;3:1-5.
6. Ma Y, Shao M, Shao X. Analysis of risk factors for intraoperative hemorrhage of cesarean scar pregnancy. Medicine. 2017;96(25):e7327.
7. Castellucci M, Scheper M, Scheffen I, et al. The development of the human placental villous tree. Anat Embryol. 1990;181:117–28.
Figures