2971
Quantitative Placental R2* Mapping on Rhesus Macaques with Ischemic Injury Model1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Comparative Biosciences, University of Wisconsin-Madison, Madison, WI, United States, 4Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 5Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Keywords: Placenta, Quantitative Imaging, R2* mapping
Placental R2* mapping is a non-invasive, quantitative biomarker with the potential to identify deficiency in uteroplacental oxygenation and blood flow, which would be of significant value in predicting and monitoring pregnancy complications. We introduce a novel thrombotic placental injury model and report mean values, histograms, and spatial distribution of baseline R2* at different gestational ages in healthy and ischemic rhesus macaque placenta based on blood oxygenation level dependent (BOLD) MR imaging. Higher increases in mean values and different spatial patterns of R2* are observed in placenta with induced ischemia.Introduction
Deficiency in uteroplacental blood flow hinders efficient nutrient, oxygen, and waste transport between maternal and fetal circulations, which can lead to or exacerbate pregnancy complications such as fetal growth restriction and preeclampsia. There is an unfulfilled need for non-invasive diagnostic tools that identify placental dysfunction early in pregnancy. Recently, T2*-weighted MRI has attracted interest as a potential marker of placental dysfunction. It can be readily obtained with standard MRI sequences, and its correlation with placental dysfunction and reference values for different field strengths and gestation have been reported1,2. In addition, quantitative T2* or R2* (=1/T2*) mapping has been introduced3,4 as the blood oxygenation level dependent (BOLD) signal correlates with placental oxygenation5. In this pilot study, we introduce a novel rhesus macaque (RM) model for inducing placental thrombi and compare their R2* results for mean values, histograms, and spatial distribution across 3 gestation time points with controls.Methods
Subjects and Interventions6 RM with singleton pregnancy were injected with 0.5 ml of Tisseel (3 RM) or saline (3 RM) as controls, all into the anterior placental disc under ultrasound guidance at ~95 days of gestational age (GA). Tisseel (Baxter Healthcare Corp) is an FDA-approved fibrin sealant used surgically to control bleeding and was used in this novel thrombotic placental damage animal model to induce thrombosis in the intervillous space and create placental ischemia and infarction.
MRI Acquisition and Processing
All subjects were imaged in right-lateral position on a 3.0-T clinical scanner (Discovery MR750 and 32-channel phased array torso coil, GE Healthcare) at GA 93.8±5.1 days, 114.2±4.2 days, and 144.3±3.8 days, hereby referred to as scans 1, 2, and 3. The treatments were performed approximately 1 day after scan 1 (~19 days before scan 2). BOLD MR data was acquired with a 3D multi-echo spoiled gradient echo sequence (first TE=1.5ms; ΔTE=2.6ms, # of TEs=16; scan time=5min27sec) that covered the entire placenta. R2* maps and water maps were generated using the method proposed by Zhu6. Placental segmentation was performed with ITK-SNAP7 based on structural images (TE=40.5ms) and water maps. Mean R2*, R2* histograms, and the spatial distribution of R2* were analyzed. The placenta discs were inspected by a pathologist after Caesarian delivery at ~155 days of gestation.
Results
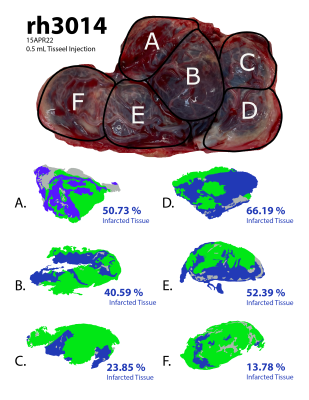
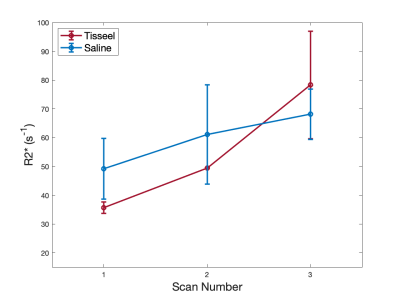
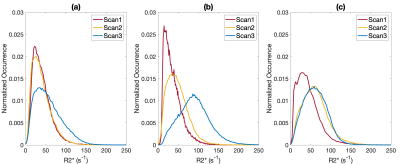
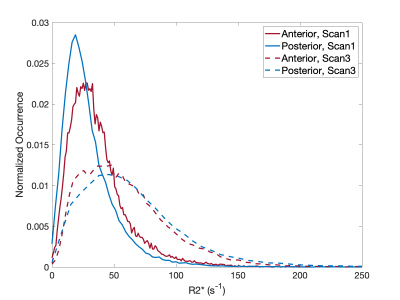
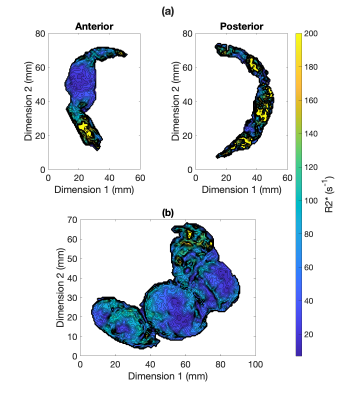
Histopathology findings in the control subjects were consistent with mature placenta (predelivery-delivery). In the Tisseel injected subjects, small fibrin thrombi were detected within the basal plate trophoblastic shell and small foci of fibrin within the subchorionic trophoblastic shell. These findings were considered histologically important but may be found in both human and macaque cases with live healthy infants. Histopathology found significant ischemic changes that multifocally affected large portions of several cotyledons in one of the Tisseel-injected subjects (rh3014) and subsequent analysis identified ischemic regions in all cotyledons (Figure 1). The presence of ischemia was also confirmed by a contrast-enhanced MRI in the baseline scan 1 before Tisseel injection. This abnormal case was analyzed outside the Tisseel group as the pathological changes likely did not result from Tisseel treatment alone. Mean placental R2* values of both groups increased with time throughout gestation, with a more significant increase observed in the Tisseel group (Figure 2). In the controls, the R2* histograms of scan 1 and 2 are rather similar, while the histogram for scan 3 is broader and shifted towards a higher mean. In the Tisseel subjects, the shift and broadening occur already at scan 2 and are much more amplified in scan 3 (Figure 3). Comparison between the anterior (injected with Tisseel) and the posterior (uninjected) placenta discs of Tisseel-treated subjects showed similar distribution (Figure 4) but varied spatial patterns in R2* values (Figure 5(a)): more spots of higher R2* values in each cotyledon are observed in the untreated disc, creating a heterogeneous pattern that is absent in the center cotyledon of the disc treated with Tisseel. The abnormal and heavily ischemic placenta shows large regions of low to medium R2* and small regions of high R2* (Figure 5(b)).Discussion and Conclusion
This study demonstrates an overall increase in R2* over gestation, with a more pronounced increase in mean values and different spatial patterns of R2* observed in Tisseel-injected placenta. The increase of mean R2* with increased gestational age shows the same trend as observed in other studies that report similar increases in R2* (or equivalent decreases in T2*3,4) and indicates a decrease in oxygenation of placental blood with gestation. The animals injected with Tisseel experienced a higher increase in R2*, as observed in other studies in the presence of pathologies8,9. Based on the local coagulation effects of Tisseel, the absence of regions of higher R2* values in the anterior disc compared to the posterior disc suggests a potential ischemia detection using R2* maps. The results of this pilot study are encouraging to further pursue this novel thrombotic placental damage animal model as well as R2* analysis. Placental dysfunction was much more severe in one subject but likely caused by a coinciding condition, yet served as a placenta dysfunction traceable by R2*. Future work will include more subjects, a detailed local comparison of R2* and histopathology, and may investigate the effects of earlier interventions.Acknowledgements
We gratefully acknowledge GE Healthcare for research support of UW-Madison, and funding support from NIH-NICHD (R01HD103443).References
1. Sørensen, A., Hutter, J., Seed, M., Grant, P.E. and Gowland, P. (2020), T2*-weighted placental MRI: basic research tool or emerging clinical test for placental dysfunction?. Ultrasound Obstet Gynecol, 55: 293-302. https://doi.org/10.1002/uog.208552.
2. Schabel, M. C., Roberts, V., Gibbins, K. J., Rincon, M., Gaffney, J. E., Streblow, A. D., Wright, A. M., Lo, J. O., Park, B., Kroenke, C. D., Szczotka, K., Blue, N. R., Page, J. M., Harvey, K., Varner, M. W., Silver, R. M., & Frias, A. E. (2022). Quantitative longitudinal T2* mapping for assessing placental function and association with adverse pregnancy outcomes across gestation. PloS one, 17(7), e0270360. https://doi.org/10.1371/journal.pone.02703603.
3. Armstrong, T., Liu, D., Martin, T., Masamed, R., Janzen, C., Wong, C., Chanlaw, T., Devaskar, S. U., Sung, K., & Wu, H. H. (2019). 3D R2* mapping of the placenta during early gestation using free-breathing multiecho stack-of-radial MRI at 3T. Journal of magnetic resonance imaging: JMRI, 49(1), 291–303. https://doi.org/10.1002/jmri.262034.
4. Huen, I., Morris, D. M., Wright, C., Parker, G. J., Sibley, C. P., Johnstone, E. D., & Naish, J. H. (2013). R1 and R2 * changes in the human placenta in response to maternal oxygen challenge. Magnetic resonance in medicine, 70(5), 1427–1433. https://doi.org/10.1002/mrm.245815.
5. Schabel, M. C., Roberts, V., Lo, J. O., Platt, S., Grant, K. A., Frias, A. E., & Kroenke, C. D. (2016). Functional imaging of the nonhuman primate Placenta with endogenous blood oxygen level-dependent contrast. Magnetic resonance in medicine, 76(5), 1551–1562. https://doi.org/10.1002/mrm.260526.
6. Zhu, A., Reeder, S. B., Johnson, K. M., Nguyen, S. M., Golos, T. G., Shimakawa, A., Muehler, M. R., Francois, C. J., Bird, I. M., Fain, S. B., Shah, D. M., Wieben, O., & Hernando, D. (2020). Evaluation of a motion-robust 2D chemical shift-encoded technique for R2* and field map quantification in ferumoxytol-enhanced MRI of the placenta in pregnant rhesus macaques. Journal of magnetic resonance imaging : JMRI, 51(2), 580–592. https://doi.org/10.1002/jmri.268497.
7. Paul A. Yushkevich, Joseph Piven, Heather Cody Hazlett, Rachel Gimpel Smith, Sean Ho, James C. Gee, and Guido Gerig. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006 Jul 1;31(3):1116-28.
8. Ho, A., Hutter, J., Jackson, L. H., Seed, P. T., Mccabe, L., Al-Adnani, M., Marnerides, A., George, S., Story, L., Hajnal, J. V., Rutherford, M. A., & Chappell, L. C. (2020). T2* Placental Magnetic Resonance Imaging in Preterm Preeclampsia: An Observational Cohort Study. Hypertension (Dallas, Tex. : 1979), 75(6), 1523–1531. https://doi.org/10.1161/HYPERTENSIONAHA.120.14701
9. Ingram, E., Morris, D., Naish, J., Myers, J., & Johnstone, E. (2017). MR Imaging Measurements of Altered Placental Oxygenation in Pregnancies Complicated by Fetal Growth Restriction. Radiology, 285(3), 953–960. https://doi.org/10.1148/radiol.2017162385
Figures