2969
Intravoxel incoherent motion MRI combined with Doppler findings in predicting very low birth weight infants1Department of Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 2Department of Obstetrics & Gynecology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 3Department of Radiology, Siemens Healthineers Ltd, Shanghai, China
Synopsis
Keywords: Placenta, Perfusion
Very low birth weight infants (VLBWI) are an adverse pregnancy outcome in small for gestational age infants (SGA). We explored the value of intravoxel incoherent motion (IVIM) histogram parameters and Doppler parameters in predicting VLBWI from SGA. Compared with the SGA group, true diffusion coefficients (Dmean, D50th, D75th, D90th) and perfusion fraction (fmax) were significantly lower, and umbilical artery pulsatility index, resistance index (RI), and peak systolic velocity/end-diastolic velocity were significantly higher in the VLBWI group. A combined predictive model (D90th and RI) improved diagnostic performance and demonstrated the potential of IVIM histogram analysis for clinical diagnosis of VLBWI.Introduction
Very low birth weight infants (VLBWI) are those who weigh less than 1,500g within 1h of birth, and small for gestation age (SGA) is the severe perinatal pregnancy outcome with significant neonatal morbidity, death, and stillbirth rate1,2. Inadequate placental perfusion is the main etiology of VLBWI3. Doppler is the primary imaging method to identify abnormal placenta vascularity. However, due to its subjective nature and inability to detect microcirculation perfusion, Intravoxel Incoherent Motion (IVIM) is recommended as a complementary diagnostic modality to improve diagnostic accuracy4,5. Therefore, our study aimed to unveil the placental IVIM histogram analysis combined with Doppler for discriminating VLBWI from SGA.Materials and Methods
The institutional review board approved the retrospective research and waived the requirement for informed consent. Thirty-three pregnant women were recruited for the study. All MRI examinations were performed using a 1.5T system (MAGNETOM Aera, Siemens Healthcare; Erlangen, Germany) with a combination of a twelve-channel surface body coil and two embedded spine coils. T2-weighted HASTE sequence was as follows: TR/TE 1300ms/167ms; slice thickness 4.0mm; FOV 380mm×309mm. IVIM images were collected with a spectrum of different b-values of 0, 50, 100, 150, 200, 500, and 800 s/mm2. The scanning parameters were acquired using TR/TE 6400ms/65ms, slice thickness 5.5mm, FOV 320mm×320mm. All IVIM data were processed with FireVoxel software (CAI2R; New York University, NY, USA). The volume of interest (VOI) was drawn with appropriate size on all continuous slices by two radiologists in fetal MRI (5- and 7-years’ experience, respectively) who were blinded to patients' information. After VOI determination, histograms of IVIM parameters were derived, which were used to automatically generate the following parameters: true diffusion coefficient (D), pseudo-diffusion coefficient (D*), perfusion fraction (f), and the mean, skewness, kurtosis, maximum, minimum, 10th, 25th, 50th, 75th, and 90th percentiles of their values (Figure1). Umbilical arterial (UA) flow velocities were measured using a pulse-wave Doppler including the following parameters, umbilical artery pulsatility index (PI), resistance index (RI), and peak systolic velocity/end-diastolic velocity (S/D).Results
Thirty-three pregnant women were divided into two groups---11 with VLBWI and 22 with SGA. Demographic and clinical characteristics are described in Table1. No significant difference was found between the gestational period of the two groups at the MRI scan (p > 0.05). As exhibited in Figure2, the Dmean, D50th, D75th, D90th, and fmax for VLBWI were significantly lower than that for SGA (p<0.05). The PI, RI, and S/D of UA were statistically higher in the VLBWI group than in the SGA group (p<0.05) (Figure2). The other parameters did not show a significant difference between the two groups (p>0.05). The area under the ROC curve (AUC) of the D90th and UA RI was higher than other parameters in distinguishing VLBWI from SGA (AUC 0.847 and 0.762, respectively) (Figure3 and Table2). The combination of D90th and UA RI further improved the AUC to 0.950, which was significantly higher than single parameters in differentiating VLBWI from SGA.Discussion
In this study, we found that IVIM histogram parameters (Dmean, D50th, D75th, D90th, and fmax) were significantly lower in the VLBWI group. We speculated that the lower D and f, the more placental interstitial infarction, fibrosis, calcification, and restricted microcirculation in VLBWI6,7. This is consistent with other studies in that D and f had significant associations with low birth weight8. Additionally, the end-diastolic peak blood flow velocity slows down in pregnant women due to increased downstream resistance, which results in increased PI, RI, and S/D, which is in accordance with our findings of fetal umbilical artery in the VLBWI1. Moreover, via ROC analysis, we found that D90th and UA RI were the best parameters with the highest AUC to predict VLBWI. The area with the highest placental perfusion is represented by the value at the 90th percentile, which was still lower in the VLBWI group than in the SGA group, further illustrating the poor placental perfusion in VLBWI. RI is the blood flow resistance index of the placenta umbilical artery, indicating that pregnant women with VLBWI were affected by pathological factors, placental blood vessels increased slowly, villous vessels had fewer branches, blood volume decreased, and blood flow showed the phenomenon of low flow and high resistance9. The combined AUC of D90th and RI showed significantly higher AUC than that any single parameter. D90th and RI are both crucial parameters to consider when assessing placental perfusion insufficiency in VLBWI.Conclusion
Histogram analysis of IVIM parameters combined with Doppler parameters may serve as sensitive indicators for predicting VLBWI from SGA.Acknowledgements
This study was supported by grants from the Maternal and Child Health research project of Jiangsu province, China [grant number No. F201845].References
1. Lees CC, Stampalija T, Baschat AA, et al. ISUOG Practice Guidelines: diagnosis and management of small‐for‐gestational‐age fetus and fetal growth restriction. Ultrasound Obst Gyn. 2020;56(2):298-312. 2. Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and Very Low Birth Weight in Infants Conceived with Use of Assisted Reproductive Technology. The New England journal of medicine. 2002;346(10):731-737. 3. Sun C, Groom KM, Oyston C, Chamley LW, Clark AR, James JL. The placenta in fetal growth restriction: What is going wrong? Placenta. 2020;96:10-18. 4. Hong S, Le Y, Lio KU, Zhang T, Zhang Y, Zhang N. Performance comparison of ultrasonography and magnetic resonance imaging in their diagnostic accuracy of placenta accreta spectrum disorders: a systematic review and meta-analysis. Insights Imaging. 2022;13(1):50. 5. Rocha AS, Andrade A, Moleiro ML, Guedes-Martins L. Doppler Ultrasound of the Umbilical Artery: Clinical Application. Rev Bras Ginecol Obstet. 2022;44(5):519-531. 6. Siauve N, Hayot PH, Deloison B, et al. Assessment of human placental perfusion by intravoxel incoherent motion MR imaging. J Matern Fetal Neonatal Med. 2019;32(2):293-300. 7. Ai Z, Han Q, Huang Z, Wu J, Xiang Z. The value of multiparametric histogram features based on intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) for the differential diagnosis of liver lesions. Ann Transl Med. 2020;8(18):1128. 8. He J, Chen Z, Wen T, Xu L, Chen C, Liu P. Utility of placental diffusion-weighted magnetic resonance imaging in prenatal diagnosis of small for gestational age infants and pregnancy outcome prediction. Placenta. 2022;121:91-98. 9. James JL, Boss AL, Sun C, Allerkamp HH, Clark AR. From stem cells to spiral arteries: A journey through early placental development. Placenta. 2022;125:68-77.Figures
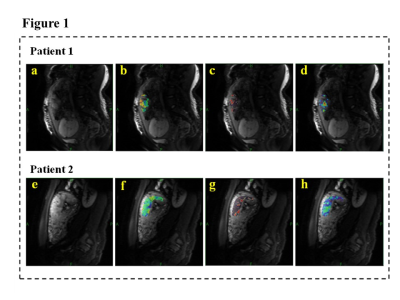
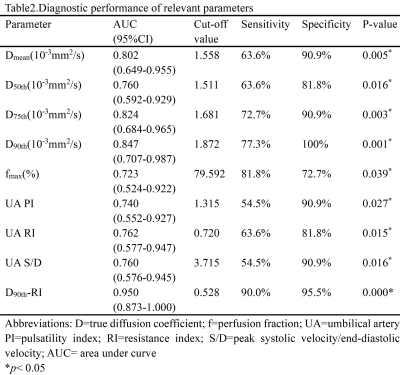
Figure1.Two examples of Intravoxel Incoherent Motion (IVIM) corresponding pseudo-color maps. Patient 1 belongs to the very low birth weight infant(VLBWI) group(a,b,c,d), and patient 2 belongs to the small for gestation age (SGA) group(d,e,f,g).
Diffusion weighted image at b-value=100mm2/s(a,e); D, true diffusion coefficient, map(b,f) ; D*, pseudo-diffusion coefficient, map(c,g); f, perfusion fraction, map(d,h)
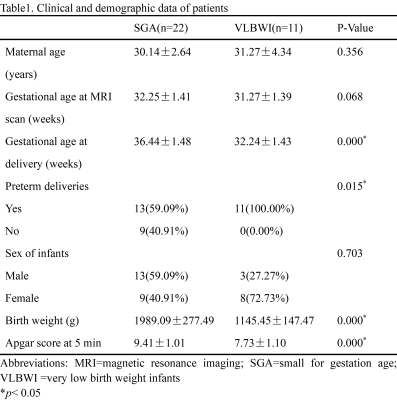
Table1. Clinical and demographic data of patients
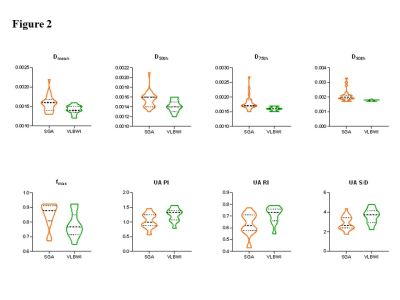
Figure2.Violin plots of placenta intravoxel incoherent motion histogram parameters and Doppler findings with statistical significance between small for gestation age (SGA) and very low birth weight infants (VLBWI) groups.
D, true diffusion coefficient; f, perfusion fraction; PI, pulsatility index; RI, resistance index; S/D, peak systolic velocity/end-diastolic velocity; UA, umbilical arterial
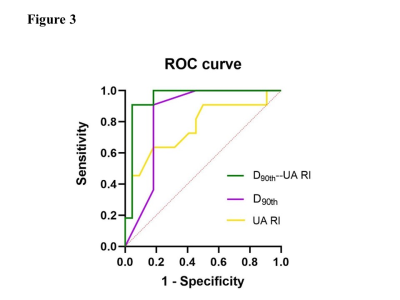
Figure3.ROC curves of true diffusion coefficient (D90th), umbilical arterial-resistance index (UA-RI), and the combined model in predicting very low birth weight infants.
D, true diffusion coefficient; RI, resistance index; UA, umbilical arterial