2954
Within Patient Contrast Adjustment Through a Self-Consistent Deep Learning Model when Imaging Near Metal: An Example in Angiography1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Center for Imaging Research, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Vessels, Metal Artifact
Multi-spectral imaging around metallic implants allows for a unique application of subject-specific deep learning applications because both high-resolution standard acquisitions and multi-spectral acquisitions are performed with significant regions of artifact-free overlap. A deep neural network can be trained in that region of artifact-free overlap within a single patient to yield a contrast transform on the multi-spectral images and achieve similar contrast to traditional acquisitions in the region of those acquisitions obscured by metal artifacts. In this proof of concept, a preliminary example of synthesizing time of flight-like contrast from a multi-spectral acquisition with vascular flow voids was shown.Introduction
Techniques for MR imaging around metallic hardware have been developed {1,2} and widely used for orthopaedic applications {3}. Other applications, however, have been sparse due to the lacking availability of relevant contrasts for non-orthopedic applications. Recently, there have been advances in metal artifact reduced diffusion and T2* weighted imaging {4,5}. Such technology offering more diverse contrasts is enabling the extension of multi-spectral imaging to neuroimaging applications where implanted devices including flow diverters, shunts, aneurysm coils and clips, skull caps, and cochlear implants cause significant artifacts from polarizing field disturbances.Here, a workflow for contrast transforms in multi-spectral acquisitions is described. It is demonstrated in angiographic imaging. In short, flow voids in the fast spin echo multi-spectral acquisition are used with the concepts of fast blood imaging {6} to achieve negative vascular contrast. Then, a deep neural network, trained and validated on a per-exam basis, is used to further highlight vasculature with a contrast similar to standard time of flight techniques {7}.
Methods
A human participant gave written informed consent and was imaged under a protocol approved by the local institutional review board. The participant was imaged on a 3.0T MRI system with a 3D multi-spectral (3D-MSI) acquisition {1} to achieve T1, T2, and proton density weighted images (T1: TR/TE 900/8.5 ms, echo train length 12; T2: TR/TE 5000/78.1 ms, echo train length 40; PD: TR/TE 3500, 28.5 ms, echo train length 30; all FOV 25.6 cm, matrix 256x256, slice thickness 4 mm, refocus flip 85 degrees), and with a standard time of flight (TOF) acquisition (TR/TE 24.0/3.4 ms, flip angle 15 degrees, FOV 20.0 cm, matrix 400x400, slice thickness 1.0 mm).A ferroshim chip was placed at the approximate relative location of a cochlear implant magnet, affixed to the 48-channel head coil, and separated from the participant with a dielectric pad. This arrangement safely yields a significant disruption in the polarizing field homogeneity, and simulates the artifact arising from MRI-conditional cochlear implant magnets which are known to yield signal voids covering up to half of a patient's brain.
Following acquisition and registration across acquired contrasts, a subject-specific U-Net {9} was trained to highlight the vasculature. The U-Net (4 encoder/decoder blocks--64, 128, 256, 512 filters--and a 1024 filter bottleneck) ingested the multi-contrast 3D-MSI images (with augmentation of left-right, anterior-posterior, shift, scale, rotate, piecewise affine, grid distortion, and optical distortion applied on a subset of images in each training epoch) and was trained to predict a thresholded version of the TOF acquisition with training masked to artifact free regions of the TOF acquisition. Slices of the volumes were randomly split into train and validation datasets, and, following 100 epochs of training wherein the soft margin loss function was evaluated within a mask of artifact-free regions of the TOF acquisition, the model with best performance on the validation dataset was used to infer a vascular image from the full set of acquired multi-spectral data--including in the region of signal loss of the TOF angiographic image. Figure 1 depicts this training process.
Results
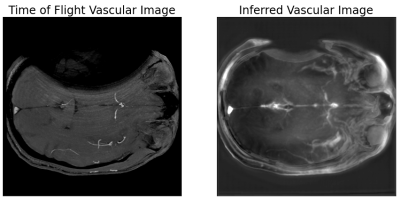
Figure 2 shows axial acquired time of flight and multi-spectral imaging inferred vascular images in a human participant at the level of the M2 component of the middle cerebral artery. While the right middle cerebral artery is visible in the time of flight acquisition, field inhomogeneity significantly disrupts the spatial encoding of the acquisition on the left half of the brain. Conversely, the multi-spectral inferred vascular image shows recovered vascular signal in both the left and right middle cerebral arteries.Discussion
Imaging in the presence of metallic implants presents a unique opportunity for patient-specific image inference optimizations. Standard, high-resolution acquisitions are performed to achieve high-quality images in regions distant from the implant while 3D-MSI acquisitions achieve lower resolution diagnostic images in the region of the implant. Thus, there are overlapping regions imaged with both standard and 3D-MSI techniques. This allows for patient-specific training of models to enhance 3D-MSI images where training occurs in regions of artifact-free image overlap. By randomly splitting the acquired volume into train and validation sets, early stopping addresses over-fitting.Here, vascular contrast, similar to time of flight angiography, was inferred from standard 3D-MSI images wherein vascular contrast is observed with flow voids. The inferred vascular image is of lower resolution than the standard time of flight acquisition, but achieves bright vessel contrast in the left middle cerebral artery which is fully obscured by artifact in the TOF acquisition. Further work to optimize input contrasts and acquisitions to minimize imaging duration and improve inference performance are ongoing.
Conclusion
The workflow of multi-spectral imaging around metallic implants allows for a unique application of subject-specific deep learning applications because both high-resolution standard acquisitions and multi-spectral acquisitions are performed with significant regions of artifact-free overlap. A deep neural network can be trained in that region of artifact-free overlap within a single patient to yield a contrast transform on the multi-spectral images and achieve similar contrast to traditional acquisitions in the region of those acquisitions obscured by metal artifacts. In this proof of concept, a preliminary example of synthesizing time of flight-like contrast from a multi-spectral acquisition with vascular flow voids was shown.Acknowledgements
This work was funded by R21EB030123.References
{1} Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med. 2009 Feb;61(2):381-90. doi: 10.1002/mrm.21856
{2} Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice Encoding for Metal Artifact Correction in MRI. Magn Reson Med. 2009 Jul;62(1):66-76. doi: 10.1002/mrm.21967
{3} Koff MF, Burge AJ, Potter HG. Clinical magnetic resonance imaging of arthroplasty at 1.5 T. J Orthop Res. 2020 Jul;38(7):1455-1464. doi: 10.1002/jor.24606. Epub 2020 Feb 4.
{4} Koch KM, Bhave S, Kaushik SS, Nencka AS, Budde MD. Multispectral diffusion-weighted MRI of the instrumented cervical spinal cord: a preliminary study of 5 cases. Eur Spine J. 2020 May;29(5):1071-1077. doi: 10.1007/s00586-019-06239-z
{5} Koch KM, Swearingen BS, Nencka AS. Multi-spectral susceptibility-weighted imaging in the presence of metallic hardware. Proc. ISMRM 2021: 2460
{6} Wedeen VJ, Meuli RA, Edelman RR, Geller SC, Frank LR, Brady TJ, Rosen BR. Projective imaging of pulsatile flow with magnetic resonance. Science. 1985 Nov 22;230(4728):946-8. doi: 10.1126/science.4059917
{7} Keller PJ, Drayer BP, Fram EK, Williams KD, Dumoulin CL, Souza SP. MR angiography with two-dimensional acquisition and three-dimensional display. Work in progress. Radiology. 1989 Nov;173(2):527-32. doi: 10.1148/radiology.173.2.2798885
{8} Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. InInternational Conference on Medical image computing and computer-assisted intervention 2015 Oct 5 (pp. 234-241). Springer, Cham.
Figures