2945
The effects of brain diffusion MRI preprocessing pipelines in a clinical study of neurodegenerative disease1Department of Radiology, Toho University, Faculty of Medicine, Tokyo, Japan, 2Department of Radiology, Juntendo University, Faculty of Medicine, Tokyo, Japan, 3Department of Neurology, Toho University, Faculty of Medicine, Tokyo, Japan
Synopsis
Keywords: Data Processing, Diffusion/other diffusion imaging techniques, Image preprocessing
The impacts of different diffusion MRI preprocessing pipelines on the statistical results of clinical studies were investigated in amyotrophic lateral sclerosis. The results illustrate the influences of popular preprocessing tools on the effect size of the disease in DKI metrics.Introduction
The preprocessing of diffusion MRI (dMRI) data is a critical step in the study workflow to correct image artifacts and to improve accuracy and precision1-3. However, there is currently a large degree of freedom in the choice of preprocessing methods1,4, likely affecting the reproducibility of studies. While preprocessing pipelines are typically tested using SNR, numerical phantoms, and scan-rescan experiments, the influence on the results of disease studies has been less investigated5. Opposite to the naïve expectations, in a study of systematic lupus erythematosus, Kornaropoulos et al.5 recently reported that the use of MPPCA denoising6 and Gibbs ringing correction7 reduced the effect size of the disease. Given the popularity of these tools in the community4, whether such reduction of sensitivity generally occurs in other situations is worth investigating. Here, we explored the impact of preprocessing pipelines in a clinical study of amyotrophic lateral sclerosis (ALS) focusing on diffusion kurtosis imaging (DKI)8 metrics. We considered ALS as appropriate to compare sensitivity to the pathology across pipelines because we can rely on the abundance of literature9,10 about the existence of motor pathway degeneration in this disease.Methods
Participants: MRI data from 23 patients with ALS (58.3±12.3 years old, disease duration 4±4 years, ALSFRS-R11 score 38±5) and 17 age- and gender-matched healthy controls (56.8±12.3 years old) were analyzed.Image acquisition: The subjects were scanned with a 3-T unit (Skyra, Siemens) using the protocol described in Koike, et al12. Two dMRI series with opposing phase encoding directions were acquired to cover two b-value shells (b = 0/700/2000 s/mm2, 15/40/80 volumes). Other parameters included: 1.7 mm isotropic voxel, 84 axial slices, TE = 89 ms, TR= 3600 ms, grappa factor 2, multiband factor 2, and partial Fourier 6/8.
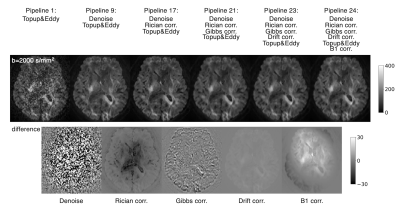
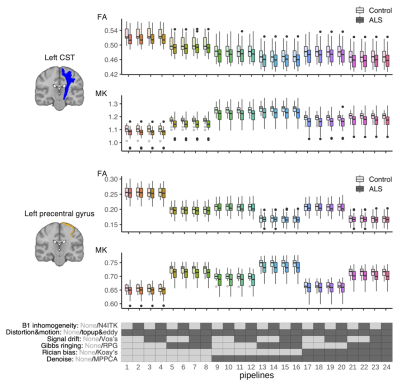
Preprocessing: The dMRI data were processed using MRtrix3 v3.0.3 and FSL v6.0.4. Based on the frequency of use in a recent survey4, the preprocessing in this work included MPPCA denoising6, Rician bias correction13, Gibbs ringing correction14, signal drift correction15, EPI distortion correction16, eddy current and motion correction17, and correction for B1 field inhomogeneities18. Pipelines with and without each of these steps were considered, resulting in 24 different pipelines (Figure 1&2). Distortion and motion correction were used for all pipelines because they were mandatory for visually-acceptable registration to anatomical T1w. Also note that Rician bias correction was used always with denoising as it depends on the noise sigma estimated by MPPCA. Anatomical T1w was processed with FreeSurfer v6.0.
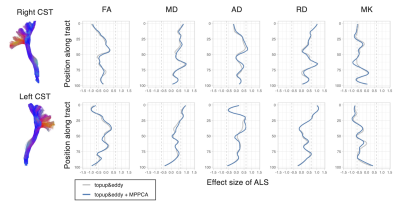
Image analyses: The DKI metrics were estimated using a constrained weighted linear least squares fit19. The ROI of the corticospinal tract (CST) and precentral gyrus was taken from the Juelich Atlas and Desikan-Killiany atlas, respectively. Tract profile analysis along the CST was performed using TractSeg20. The effects of the disease were examined by linear regression: Y = β0 + βALSXALS + βageXage + βgenderXgender + ϵ, and Cohen’s d was calculated from the t statistics21.
Results
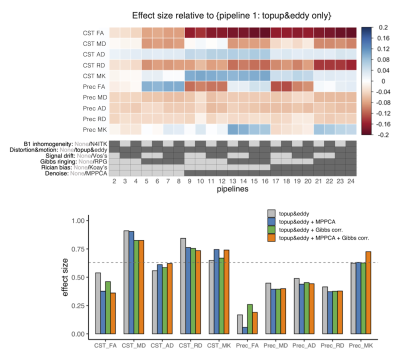
ROI-based analyses revealed that the effects of pipelines often exceed those of the disease, while the frequently reported trends like smaller kurtosis and greater diffusivities in the patients9,22,23 were consistent across pipelines (Figure 3). MPPCA and Gibbs ringing correction had the largest impacts on the values of diffusion metrics and the effect size (Figure 3&4). The largest difference in Cohen’s d between pipelines was about 0.19. The use of signal drift correction and B1 inhomogeneity correction induced only small changes.In the CST ROIs, the pipelines with MPPCA and Gibbs ringing correction yielded smaller ALS effect size in fractional anisotropy (FA), mean diffusivity (MD), and radial diffusivity (RD) compared to those without them (Figure 4), partly in agreement with Kornaropoulos et al.5. The positive impact of MPPCA was observed for mean kurtosis (MK), and in the tract profile analyses (Figure 5). Gibbs ringing correction had a positive impact on the disease effect size for MK in the precentral gyrus ROI.
Discussion & Conclusion
The differences caused by pipelines were greater than those by the disease. The dMRI metrics obtained through different pipelines cannot be compared, and a consensus for best practice in preprocessing is of critical importance to delivering clinically usable diagnostic thresholds in quantitative dMRI.The present results demonstrate the nonnegligible impacts of preprocessing on statistical results. Whether a particular preprocessing has a positive or negative impact on disease effect size depended on the target region, diffusion metric, and types of analyses. While Kornaropoulos et al.5 reported no clear benefits of MPPCA and Gibbs ringing correction regarding the effect size, we observed a positive impact on the effect size for MK, though at the cost of reduced effect size for FA, MD, and RD in ROI-based analyses. Given that MPPCA and Gibbs ringing correction improve the accuracy of DKI2, our observation may imply the disease effects were (correctly) transferred to the kurtosis term from the diffusivity part. Also, the benefits of advanced preprocessing are probably more appreciated in applications like voxel-wise or tract-profile analyses which are more sensitive to noise and artifacts than the analyses of average over the whole tract5.
Acknowledgements
This study was supported by Japan Society for the Promotion of Science (JSPS) [21K07629].References
1. Tax CMW, Bastiani M, Veraart J, Garyfallidis E, Okan Irfanoglu M. What's new and what's next in diffusion MRI preprocessing. Neuroimage. 2022;249:118830.
2. Ades-Aron B, Veraart J, Kochunov P, et al. Evaluation of the accuracy and precision of the Diffusion parameter EStImation with Gibbs and NoisE Removal pipeline. Neuroimage. 2018;183:532-543.
3. Maximov II, Alnaes D, Westlye LT. Towards an optimised processing pipeline for diffusion magnetic resonance imaging data: Effects of artefact corrections on diffusion metrics and their age associations in UK Biobank. Hum Brain Mapp. 2019;40:4146-4162.
4. Veraart J, Christiaens D, Dai E, et al. A data-driven variability assessment of brain diffusion MRI preprocessing pipelines. Proc. Intl. Soc. Mag. Reson. Med. 2022. p4983.
5. Kornaropoulos EN, Winzeck S, Rumetshofer T, et al. Sensitivity of Diffusion MRI to White Matter Pathology: Influence of Diffusion Protocol, Magnetic Field Strength, and Processing Pipeline in Systemic Lupus Erythematosus. Front Neurol. 2022;13:837385.
6. Veraart J, Novikov DS, Christiaens D, et al. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394-406.
7. Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76(5):1574-1581.
8. Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432-40.
9. Kassubek J, Müller HP. Advanced neuroimaging approaches in amyotrophic lateral sclerosis: refining the clinical diagnosis. Expert Rev Neurother. 2020;20:237-249.
10. Braak H, Brettschneider J, Ludolph AC, Lee VM, Trojanowski JQ, Del Tredici K. Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nat Rev Neurol. 2013;9:708-14.
11. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13-21.
12. Koike S, Tanaka SC, Okada T, et al. Brain/MINDS beyond human brain MRI project: A protocol for multi-level harmonization across brain disorders throughout the lifespan. Neuroimage Clin. 2021;30:102600.
13. Koay CG, Basser PJ. Analytically exact correction scheme for signal extraction from noisy magnitude MR signals. J Magn Reson. 2006;179:317-22.
14. Lee HH, Novikov DS, Fieremans E. Removal of partial Fourier-induced Gibbs (RPG) ringing artifacts in MRI. Magn Reson Med. 2021;86:2733-2750.
15. Vos SB, Tax CM, Luijten PR, et al. The importance of correcting for signal drift in diffusion MRI. Magn Reson Med. 2017;77:285-299.
16. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870-88.
17. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-1078.
18. Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310-20.
19. Veraart J, Sijbers J, Sunaert S, et al. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. Neuroimage. 2013;81:335-346.
20. Wasserthal J, Neher P, Maier-Hein KH. TractSeg - Fast and accurate white matter tract segmentation. Neuroimage. 2018;183:239-253.
21. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591-605.
22. Chen HJ, Zhan C, Cai LM, et al. White matter microstructural impairments in amyotrophic lateral sclerosis: A mean apparent propagator MRI study. Neuroimage Clin. 2021;32:102863.
23. Welton T, Maller JJ, Lebel RM, et al. Diffusion kurtosis and quantitative susceptibility mapping MRI are sensitive to structural abnormalities in amyotrophic lateral sclerosis. Neuroimage Clin. 2019;24:101953.
Figures