2940
Deep learning of 3D T2-weighted MRI provides support for arachnoid granulation hypertrophy in patients with Parkinson’s disease1Department of Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Department of Radiology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Keywords: Data Analysis, Neurodegeneration
We apply novel deep learning algorithms using non-contrasted T2-weighted MRI to test hypotheses regarding arachnoid granulation (AG) hypertrophy in patients with Parkinson’s disease (PD). Using this method, we identify AGs protruding into the superior sinus lumen, which may serve as a site of CSF egress implicated in the neurofluid clearance system. Results suggest a significant increase in AG volume in the parietal and frontal lobes of PD participants compared to age-matched healthy controls, potentially indicative of reduced neurofluid clearance efficiency secondary to macromolecular aggregation along the CSF circuit.Introduction
The overall goals of this work are to (1) validate novel MRI-based deep learning algorithms for the segmentation of arachnoid granulation (AG) volume and (2) apply these algorithms to test fundamental hypotheses regarding differences in AG volume in older adults with and without Parkinson’s disease (PD). PD is a neurodegenerative disorder with unknown cause characterized by loss of dopaminergic neurons, alpha-synuclein and often beta-amyloid proteinopathy, and manifestation of motor and cognitive decline. While dopaminergic mechanisms in PD have been extensively characterized, there is emerging evidence that neurofluid circulation impairment, through either bulk CSF or recently-proposed glial-lymphatic circuits1, may have implications for protein retention and symptomatology in PD2,3,4. Although it is difficult to measure this directly with existing methods, the functionality of the arachnoid granulations (AGs), serving as one-way valves for CSF resorption into dural venous sinuses5,6, is fundamental to neurofluid circulation. While volumetric changes in AG have been investigated in healthy aging7, these metrics have not been analyzed in patients with PD, largely owing to a lack of robust technologies for characterizing these structures reliably in vivo. To address this limitation, we developed and validated a novel deep learning algorithm that enables quantification of AG distribution and volume non-invasively in vivo from 3D T2-weighted scans8. We subsequently apply this method to understand relationships between AG hypertrophy and neurodegeneration in older adults with and without PD.Methods
All (n=75) participants (PD: n=25; healthy: n=50) provided informed consent and were scanned at 3T MRI (Philips) using body coil transmission and phased-array 32-channel SENSE reception. MRI sequence. 3D T2-weighted sagittally acquired VISTA with TR/TE=2500/331 ms and spatial resolution = 0.78x0.78x0.78 mm. Preprocessing. T2-weighted MRIs were corrected for inhomogeneity field9 and aligned to the MNI template using affine registration10. Algorithm. The proposed AG segmentation method is based on a cascaded U-Net model11 with 3D patches as inputs (96x64x64voxels). Deep-learning models were trained using 50 manually delineated scans. Analysis. AGs were manually delineated along the superior sagittal sinus (SSS) under the supervision of a board certified neuroradiologist (experience=13 years). Delineation of prefrontal, frontal, parietal, and occipital regions were identified on the MNI template and projected to the native space. The prefrontal and frontal regions were distinguished by a plane crossing through the pituitary gland and the rostrum of the corpus callosum, such that the prefrontal region lies ventral and the frontal region dorsal to the plane. The parietal region was delineated from the frontal region using the central sulcus and extends to the parietal-occipital fissure; the occipital region was delineated from the parietal-occipital fissure to the most posterior portion. Hypothesis testing. Dice-Sørensen coefficient (DSC), sensitivity, and specificity in a 6-fold cross validation iterated 6 times. Pearson correlations were calculated to evaluate correlation between estimated volume using deep-learning and ground truth volumes using manual delineation. A multi-linear regression model was applied with AG volume (total, prefrontal, frontal, parietal, and occipital) as the independent variable. Sex and pathological group (i.e., healthy or PD) served as dependent variables. Statistical significance was computed using ANOVA one way test (significance criteria, p<0.05).Results
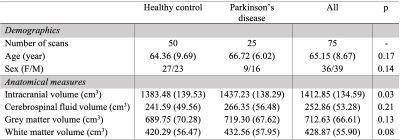
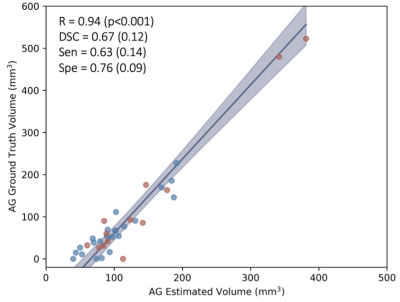
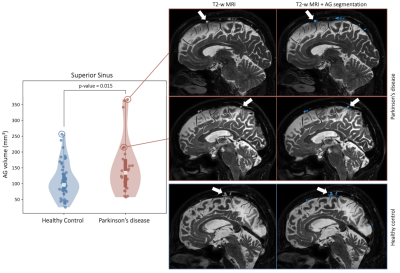
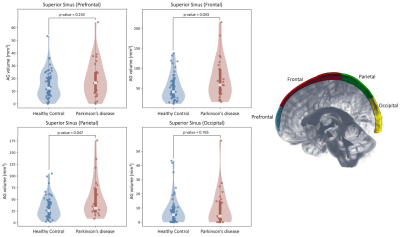
Table 1 summarizes demographic information for healthy and PD participants, with estimation of statistical differences; difference in anatomical volumetrics was led by unbalanced sex proportion. Validation. Manual delineation yielded moderate DSC of 0.67, and strong correlation with estimated volumes (Figure 1; R=0.94, p-value<0.001). Group comparison. AG volume showed a significant increase in PD compared to healthy participants (p=0.017); males were also observed to have larger AG volume compared to females (Figure 2; p=0.019). Figure 3 displays the topography of the AG distribution along the SSS. AG volumes were found to be significantly larger than volumes in healthy participants in the frontal (p=0.043) and parietal (p=0.047) regions, with inconclusive findings in the prefrontal (p=0.234) and occipital (p=0.765) regions.Discussion and Conclusions
We developed and validated, using manual delineation, a method for quantifying AG volume and topography along the sagittal sinus automatically from non-contrasted 3D T2-weighted MRI. This method extends prior work pertaining to the automatic delineation of parasagittal dural space and dural sinus anatomy to provide a more complete perspective on anatomical features of neurofluid egress along the venous macro-vasculature. Findings support a significant correlation between manual tracing and automatic AG segmentation. Importantly, this work was designed to utilize only 3D non-contrasted T2-weighted MRI at the spatial resolution of 0.78 mm (isotropic), which should allow for these metrics to be quantified from the wide number of protocols that use this common anatomical sequence. A limitation is that the method tends to overestimate small AG (typically under 5 mm3) and underestimate large AG (typically over 150 mm3), which can lead to a reduced power of detection for groups that are on the opposite side of the AG volume spectrum. Finally, we applied this method to delineate AG volume in PD and age-matched healthy adults. Results suggest a significant increase in AG volume in the frontal and parietal regions of PD participants compared to age-matched healthy controls. Such hypertrophy of AGs has recently been suggested for healthy aging7, potentially indicative of reduced clearance efficiency secondary to macromolecular aggregation along the CSF circuit and may induce flow-limiting stenosis of the dural sinuses.Acknowledgements
This study has been supported in part by the National Institute of Health (NIH) through grant numbers K24-AG064114, R01-NR015079, and R01-AG062574, the Department of Defense (DoD) W81XWH-19-1-0812, and the Huntington's Disease Society of America (HDSA) HD Human Biology Project Fellowship.References
1. Braak et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24(2), 197-211 (2003).
2. Ding, X. B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat Med, 27, 411–418 (2021).
3. Donahue, E. K. et al. Global and Regional Changes in Perivascular Space in Idiopathic and Familial Parkinson's Disease. Movement Disorders, 36(5), 1126-1136 (2021).
4. McKnight et al. Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Parkinsonism & Related Disorders, 89, 98-104 (2021).
5. Bohr, T. et al. The glymphatic system: Current understanding and modeling. iScience, 25(9), (2022).
6. McKnight, D. et al. The regulation of cerebral spinal fluid flow and its relevance to the glymphatic system. Curr Neurol Neurosci Rep, 20, (2020).
7. Radoš, M. et al. No arachnoid granulations-no problems: Number, size, and distribution of arachnoid granulations from birth to 80 years of age. Frontiers in Aging Neuroscience. (2021).
8. Hett, K. et al. Parasagittal dural space and cerebrospinal fluid (CSF) flow across the lifespan in healthy adults. Fluids Barriers CNS 1–33 (2022).
9. Tustison, N. J. et al. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
10. Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
11. Ronneberger, O., Fischer, P. & Brox, T. U-net: Convolutional networks for biomedical image segmentation. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 9351, 234–241 (2015).
Figures