2901
Improved perfusion-weighted images at 7T combining pTx and low B1+ Adiabatic pulses1Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 2Computational Cognitive Neuroscience and Neuroimaging, Netherlands Institute for Neuroscience, Amsterdam, Netherlands, 3Radiology, Leiden University Medical Center, Leiden, Netherlands
Synopsis
Keywords: Data Acquisition, High-Field MRI
A FAIR acquisition at 7T, using a TR-FOCI pulse for background suppression, was set up on a pTx (32Rx8Tx) system and on the standard (32Rx2Tx) system. CBF-imaging results were compared in terms of CBF-weighted signal intensities and drop-off with increasing thickness of the slab-selective inversion slab. The higher and more homogeneous B1 of the pTx system translated to higher CBF-weighted signal intensities. Both setups allowed functional mapping of the hand region in the motor cortex in approximately 5 minutes.Introduction
Arterial spin labeling (ASL) is one of the most versatile MRI techniques. ASL provides a specific tool to measure non-invasive Cerebral Blood Flow (CBF) and, as an alternative, a more physiological informing complement to BOLD fMRI (1,2). However, CBF measurements using ASL suffer from an intrinsically lower signal-to-noise ratio (SNR), reduced temporal resolution, limited brain coverage due to T1 relaxation, and increased power deposition (1). Ultra-high field (UHF) ASL promises to be advantageous since the perfusion SNR increases with the static field, and T1 relaxation times become longer (3). However, UHF ASL implementations are not trivial. Challenges such as B0 and B1 inhomogeneities need to be carefully addressed (4). For example, several adiabatic inversion pulses have been specifically proposed for FAIR-ASL to mitigate the B1+ inhomogeneity (5,6). In addition, for pCASL, the labeling plane was raised from the neck to the bottom of the cerebrum to take advantage of the higher B1+ and the lower SAR (7). Another possible solution for a more homogeneous transmit field is parallel transmission (pTx) technology; pTx capabilities rely on using the additional degree of freedom of the multiple channels to better control the B1+ field. Strategies such as B1-shimming or tailored RF pulses (8,9) were already optimized and evaluated in ASL. When applied to FAIR, pTx might provide a wider bolus-width by allowing labeling lower in the brain/neck area. Unfortunately, pTx systems are not yet widely available. In the present study, we used a pTx setup (32Rx8Tx) in combination with a TR-FOCI pulse and compared the results to the 32Rx2Tx coil on the standard 2-channel system using the same sequence and RF pulsesMethods
Four healthy volunteers (1 female) participated in the study. Imaging was performed on a 7T scanner (Philips) using 32Rx2Tx and 32Rx8Tx rf-coils (both Nova Medical). The pTx system was used with a close-to-circularly polarized-mode achieved by B1-shimming over the entire brain of a separate group of volunteers. Flow-alternating-inversion-recovery (FAIR) labeling was implemented using time-resampling frequency-offset-corrected-inversion (TR-FOCI) RF-pulses (5). Pre- and post-saturation modules were included to saturate the imaging volume. We employed two non-selective TR-FOCI RF-pulse for the background suppression at 1200 and 1830ms. The acquisition consisted of a multi-slice, single-shot EPI (resolution:3×3mm2, 13 slices of 3mm, TI=2s, TR=5500ms). The inter-slice duration was 65ms. To measure the temporal width of the bolus of labeled spins, FAIR data was acquired from three volunteers with a variable thickness of the slab-selective inversion pulse of 6cm, 9cm, and 12cm larger than the volume-of-interest for both coil setups. For the pTx system, a max B1 value of 18μT was used, while for the 2Tx a max B1 of 12μT was set to accommodate amplifier limitations. An extra 6cm slab dataset was acquired at the pTx system with B1=12μT. Additionally, a multi-inversion delay scan with the same EPI readout and slab positioning was also acquired to estimate inversion efficiency. In the fourth participant, a 33-volume pair time course (6 minutes) was acquired with the 6cm labeling slab protocol during a functional task on both the 2-channel and 8-channel systems. DREAM B1-maps (10) and 0.8mm MPRAGE anatomical data (11) were also acquired for all volunteers.Results & Discussion
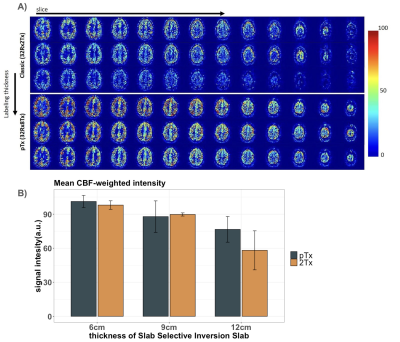
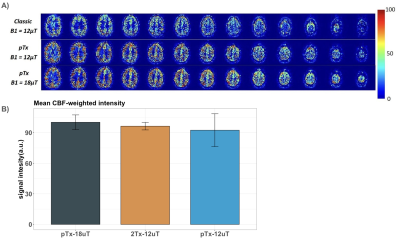
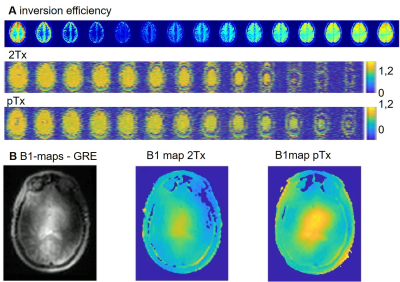
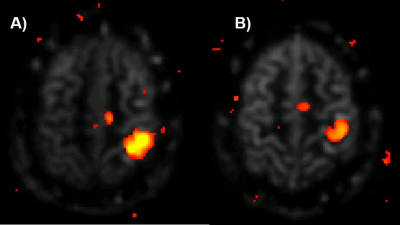
Figure 1 shows CBF-weighted images from a single participant, depicting data from three different thicknesses of the slab-selective inversion pulse and the differences between the Classic system (32Rx2Tx) and the pTx system (32Rx8Tx). The increase in the thickness of the slab-selective inversion, resulted in an intensity reduction in the CBF-weighted images in both Classic and pTx, with a significant reduction of the classic system compared to the pTx. Results were consistent across participants (Fig.1B). Figure 2 shows CBF-weighted images from a single participant depicting the minimal difference between different B1 values from the pTx and the difference between both pTx with the 2Tx classic system. The difference between 2Tx and pTx does not appear to be driven by the reference B1 value, but rather by the rf-profile of the coils. Figure 3 shows the inversion efficiency of both coils. The inversion efficiency maps at the level of the imaging slab are comparable between 2Tx and pTx systems (Fig 3A), but the DREAM B1 maps do differ lower in the brain, where the label signal arises (Fig 3B). The functional results in Figure 4 show comparable responses for this individual participant, which was first scanned on the 2Tx system. For quantitative comparisons, counterbalancing of acquisition is required to avoid order effects. Nonetheless, it is clear that CBF-based functional maps can be successfully acquired at 7T with this <6-min protocol.Conclusion
The FAIR sequence with TR-FOCI yielded high image quality on both 2Tx and pTx systems. Upon increasing the thickness of the slab-selective inversion slab, signal decreases were smaller in the pTx system, reflecting better coil performance.Acknowledgements
No acknowledgement found.References
1. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73:102–116.
2. Hernandez-Garcia L, Aramendia-Vidaurreta V, Bolar DS, et al. Recent Technical Developments in ASL: A Review of the State of the Art. Magn. Reson. Med. 2022;88.
3. Ivanov D, Gardumi A, Haast RAM, Pfeuffer J, Poser BA, Uludağ K. Comparison of 3T and 7T ASL techniques for concurrent functional perfusion and BOLD studies. NeuroImage 2017;156:363–376.
4. Teeuwisse WM, Webb AG, van Osch MJP. Arterial spin labeling at ultra-high field: All that glitters is not gold. Int. J. Imaging Syst. Technol. 2010;20:62–70.
5. Hurley AC, Al-Radaideh A, Bai L, et al. Tailored RF pulse for magnetization inversion at ultrahigh field. Magn. Reson. Med. 2010;63:51–58.
6. Ordidge RJ, Wylezinska M, Hugg JW, Butterworth E, Franconi F. Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy. Magn. Reson. Med. 1996;36:562–566.
7. Zuo Z, Wang R, Zhuo Y, Xue R, Lawrence KSS, Wang DJJ. Turbo-FLASH Based Arterial Spin Labeled Perfusion MRI at 7 T. PLOS ONE 2013;8:e66612.
8. Tong Y, Jezzard P, Okell TW, Clarke WT. Improving PCASL at ultra-high field using a VERSE-guided parallel transmission strategy. Magn. Reson. Med. 2020;84:777–786.
9. Wang K, Ma SJ, Shao X, et al. Optimization of pseudo-continuous arterial spin labeling at 7T with parallel transmission B1 shimming. Magn. Reson. Med. 2022;87:249–262.
10. Nehrke K, Versluis MJ, Webb A, Börnert P. Volumetric B1 (+) mapping of the brain at 7T using DREAM. Magn. Reson. Med. 2014;71:246–256.
11. Oliveira ÍAF, Roos T, Dumoulin SO, Siero JCW, van der Zwaag W. Can 7T MPRAGE match MP2RAGE for gray-white matter contrast? NeuroImage 2021;240:118384.
Figures