2893
Preoperative MRI-based radiomic-clinical nomogram to predict residual tumor for advanced high-grade serous ovarian carcinoma1Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 2Department of Research and Development, Shanghai United Imaging Intelligence Co., Ltd, Shanghai, China, 3GE Healthcare, Beijing, China, 4Department of Radiology, Jinshan Hospital, Fudan University, Shanghai, China, 5Department of Radiology, Fudan University Shanghai Cancer Center, Shanghai, China
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Radiomics, Ovarian carcinoma, Residual tumor prediction
Residual tumor (RT) status is associated with the prognosis and survival rate of patients with high-grade serous ovarian carcinoma (HGSOC). However, current RT status prediction approach through laparoscopy has disadvantages of invasiveness, high cost and incidence of tumor metastases. In this study, we proposed a radiomic-clinical nomogram, based on multiple-sequence MRI combined with score of abdominal metastases and clinical markers, for preoperative prediction of RT status. We demonstrated that the radiomic-clinical nomogram had satisfactory prediction performance in all cohorts (AUC = 0.900-0.936). The clinical application value of the nomogram was further confirmed by decision curves.Background
Ovarian cancer (OC), the second most common female genital cancer,1 is one of the deadliest gynecologic malignancies globally.2 High-grade serous ovarian carcinoma (HGSOC) represents the most prevalent histologic subtype of OC.3 HGSOC tends to be diagnosed at an advanced stage, leading to high disease recurrence and mortality rates.4Currently, standard treatment for HGSOC patients remains primary debulking surgery (PDS), followed by platinum-based chemotherapy.5 PDS aims to achieve complete resection, leaving no residual tumor (RT). Studies have demonstrated that RT status is a crucial prognostic factor for HGSOC. An inverse relationship between extent of RT and patient survival has been reported.6-8 Patients with R0 resection at PDS had the best prognosis in randomized trials.9-10
Clinically, RT status was predicted by laparoscopy and preoperative imaging. Laparoscopy has precise diagnosis performance but with disadvantages of invasiveness, high cost and incidence of tumor metastases.11-13 Radiomics, utilizing high-throughput selection of features from imaging data, has proven to be highly effective in improving diagnostic precision, assessing treatment response, and predicting prognosis.14-16 Several predictive models were developed based on CT and MRI.17-20 While there were radiomics models for RT prediction in advanced OC,21-23 they had limitations such as the lack of abdominal metastases information or external validation. Therefore, a more comprehensive and generalize method should be developed for RT prediction.
Therefore, in this study, a radiomics model based on multiple-sequence MRI of pelvic tumors combined with score of abdominal metastases and clinical markers were developed to non-invasively predict RT in HGSOC preoperatively. Additionally, an inclusive nomogram that incorporated radiomics signatures and clinical predictors was established to provide a preoperative assessment of RT risk in HGSOC patients.
Methods
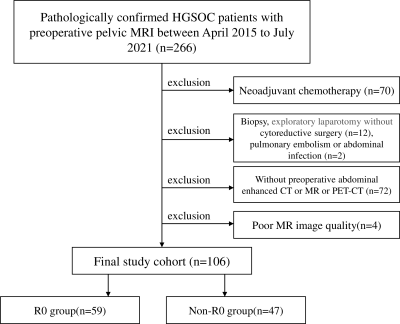
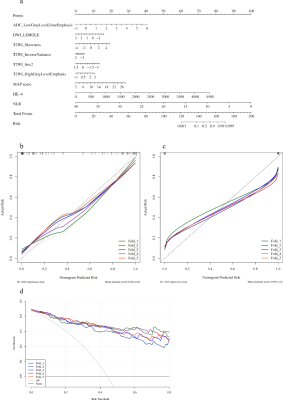
This retrospective two-center study was approved by our institutional review boards (No. B2021-324; No.2004216-24). 128 patients were enrolled in this study (Figure 1). Clinical data including age and laboratory findings such as serum CA125, HE-4 level, and NLR were obtained. Metastases in abdomen and pelvis (MAP) of HGSOC patients were evaluated and scored based on preoperative abdominal and pelvic enhanced CT and/or MR and/or PET-CT. Patients were grouped into R0 resection (RT ≤ 1 cm) and none R0 resection (RT > 1 cm) group.24MRI examinations were performed using one 1.5T and two 3.0T scanners. DWI and T2WI were acquired. Manual segmentation of tumors was performed based on T2WI using ITK-SNAP (www.itksnap.org). 258 radiomic features were extracted from T2WI, DWI and ADC images, using PyRadiomics,25 including: (1) first-order statistics features; (2) shape-based features; (3) GLCM; (4) GLSZM; (5) GLRLM; (6) GLDM. Finally, LASSO classifier was used to select the most relevant features, and to build a single-parameter radiomic model for RT status prediction. Furthermore, 6 radiomics features were selected using multivariate logistic regression, and the radiomic-clinical nomogram (Figure 3a) was developed by combining 3 clinical independent predictors.
Statistical analyses were performed using SPSS 20 (IBM Corporation, Armonk, NY) or R (https://www.Rproject.org) software. Differences of clinical and imaging features between groups were compared by Mann-Whitney U test or chi-square test. The performance of radiomics model was assessed using ROC analysis. The AUC, sensitivity, specificity, accuracy, F1-score, and precision at the Youden index were then calculated. The comparison of predictive efficacy among models was evaluated by DCA analysis. The calibration curve was provided to evaluate the agreement between true and predicted outcomes of RT status using the HL test.
Results
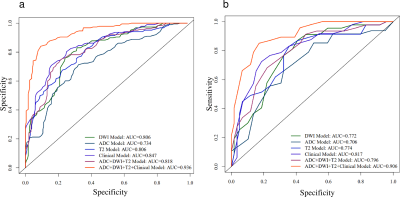
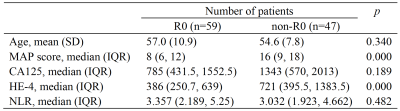
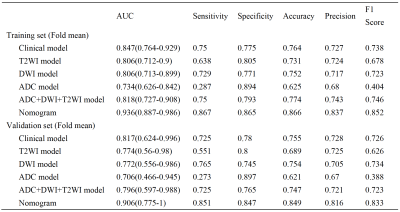
The patient clinical predictors are presented in Table 1. The MAP scores were significantly different between two groups. The HE-4 level was significantly different only in the training cohort. MAP score (p = 0.002; OR = 6.651), HE-4 level (p = 0.001; OR = 15.435) and NLR (p = 0.008; OR = 0.026) were independent predictors of RT status.The ROC results and predictive performance of each model are shown in Figure 2 and Table 2. The combination model of T2WI, DWI and ADC showed similar performance in both training (AUC = 0.727-0.908) and validation cohort (AUC = 0.597-0.988). The clinical model performed better than single MR sequence models. In the separate validation cohort, T2WI model, ADC model and the combination model (AUC = 0.825-0.858) showed better performance than the clinical model (AUC = 0.708). The radiomic-clinical nomogram performed best in predicting RT status of HGSOC in both the training cohort (AUC = 0.936; accuracy = 0.866; sensitivity = 0.867; specificity = 0.865), cross validation cohort (AUC = 0.906; accuracy = 0.849; sensitivity = 0.851; specificity = 0.847) and the separate validation cohort (AUC = 0.900; accuracy = 0.818; sensitivity = 0.800; specificity = 0.833). Calibration curves (Figure 3b, c) of the radiomic-clinical nomogram exhibited satisfactory predictive performances, and were aligned with the actual RT status. The HL test showed that data had goodness of fit. Decision curve (Figure 3d) of the nomogram showed a higher overall net benefit than that assuming all patients have R0 resection.
Conclusion
The identified radiomics features extracted from primary masses can assist in predicting RT status of HGSOC patients. The radiomic-clinical nomogram showed an excellent predictive performance in preoperative RT prediction. The MAP score demonstrated the incremental value of clinical predictors for RT estimation, although further external validation is required before its wide clinical application.Acknowledgements
No acknowledgement found.References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67:7-30.
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3):209-249.
3. Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: An analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol 2016; 34(24):2888-98.
4. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2017; 41:3-14.
5. Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 2006; 103(2):559-64.
6. Chang SJ, Bristow RE, Ryu HS. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann Surg Oncol 2012; 19(13):4059-67.
7. du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer 2009; 115(6):1234-44.
8. Fagotti A, Vizzielli G, Fanfani F, et al. Introduction of staging laparoscopy in the management of advanced epithelial ovarian, tubal and peritoneal cancer: impact on prognosis in a single institution experience. Gynecol Oncol 2013; 131(2):341-6.
9. Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015; 386(9990):249-57.
10. Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363(10):943-53.
11. van de Vrie R, Rutten MJ, Asseler JD et al. Laparoscopy for diagnosing resectability of disease in women with advanced cancer. Cochrane Database Syst Rev 2019; 3(3):CD009786.
12. Harrison RF, Cantor SB, Sun CC, et al. Cost-effectiveness of laparoscopic disease assessment in patients with newly diagnosed advanced ovarian cancer. Gynecol Oncol 2021; 161(1):56-62.
13. Passot G, Dumont F, Goere D, et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br J Surg 2018; 105(6):663-667.
14. Qian L, Ren J, Liu A et al. MR imaging of epithelial ovarian cancer: a combined model to predict histologic subtypes. Eur Radiol 2020; 30:5815-5825.
15. Peng H, Dong D, Fang MJ et al. Prognostic value of deep learning PET/CT-based radiomics: Potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res 2019; 25:4271-4279.
16. Zhang H, Mao Y, Chen X et al. Magnetic resonance imaging radiomics in categorizing ovarian masses and predicting clinical outcome: A preliminary study. Eur Radiol 2019; 29:3358-3371.
17. Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol 2014; 134(3):455-461.
18. Feng Z, Wen H, Jiang Z, et al. A triage strategy in advanced ovarian cancer management based on multiple predictive models for R0 resection: A prospective cohort study. J Gynecol Oncol 2018; 29(5): e65.
19. Qayyum A, Coakley FV, Westphalen AC, Hricak H, Okuno WT, Powell B. Role of CT and MR imaging in predicting optimal cytoreduction of newly diagnosed primary epithelial ovarian cancer. Gynecol Oncol 2005;96(2):301-306.
20. Axtell AE, Lee MH, Bristow RE, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoredcution in patients with advanced ovarian cancer. J Clin Oncol 2007; 25(4):384-389.
21. Li H, Zhang R, Li R, et al. Noninvasive prediction of residual disease for advanced high-grade serous ovarian carcinoma by MRI-based radiomic-clinical nomogram. Eur Radiol 2021; 31(10):7855-7864.
22. Lee EYP, An H, Perucho JAU et al. Functional tumour burden of peritoneal carcinomatosis derived from DWI could predict incomplete tumour debulking in advanced ovarian carcinoma. Eur Radiol 2020; 30:5551-5559.
23. Gemer O, Gdalevich M, Ravid M, et al. A multicenter validation of computerized tomography models as predictors of non-optimal primary cytoreduction of advanced epithelial ovarian cancer. Eur J Surg Oncol 2009; 35(10):1109-1112.
24. Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol 2017; 145(1):27–31.
25. van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res 2017; 77(21): e104-e107.
Figures