2892
The Impact of Compressed Sensing on Radiomics of Fast Anatomical MRI of Rat Brain at 7T1Institute of Biomedical Engineering, Boğaziçi University, İstanbul, Turkey, 2Targeted Therapy Technologies Experimental Animal Imaging Laboratory, Boğaziçi University, İstanbul, Turkey, 3Department of Biomedical Engineering, İstinye University, İstanbul, Turkey
Synopsis
Keywords: Image Reconstruction, Preclinical, Radiomics
This study aims to investigate the effect of compressed sensing reconstruction on radiomics parameters of rat brain MRI at 7T. T2 weighted MRI of rat brain were randomly under-sampled with R=2.2 and reconstructed using compressed sensing. Brains were segmented after registration to a Wistar rat brain atlas. The radiomics of original and accelerated MRI data were compared in seven scans of rat brains using a Wilcoxon signed-rank test. Stable radiomics features were identified using intraclass correlation coefficients. The findings of this study indicated that not all radiomics features of rat brain were robust to compressed sensing acceleration.Introduction
Preclinical rat brain imaging experiments are performed under anesthesia, which might result in prominent side effects, including respiratory depression with reduced respiration rate, hypercapnia and acidosis.1 Accelerating preclinical MRI is of interest to perform more scans within the time limits of animal handling safety regulations. Compressed sensing (CS) has been extensively used for accelerating MRI with low SNR penalty,2 and it has been applied for fast functional MRI,3 and dynamic susceptibility contrast (DSC) MRI4 in rats. Radiomics is an emerging field that extracts a large number of quantitative and minable features from MR images.5 Through combination of large amount of quantitative features with machine learning and statistical methods, radiomics aims to support clinical decision making.6 Despite its promise, the radiomics features present high variability in response to small changes in pre-processing,7 segmentation,8 and reconstruction techniques.9 The purpose of this study was to evaluate the robustness of radiomics features of anatomical brain MRI of rats after compressed sensing (CS) data acquisition and reconstruction at 7T.Methods
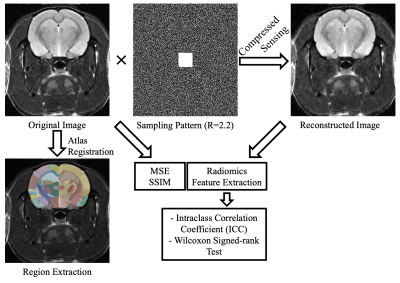
Seven MRI scans of female Wistar rats were acquired on a 7T MRS*DRYMAG 7017 Powerscan PET/MR scanner (MR Solutions, Guildford, UK) at multiple time points from ages of four to eight months old. During MRI data acquisition, anesthesia, oxygen and isoflurane gas (up to 2%) mixture was administered to the rats through a nose cone. The brain imaging protocol included T2-weighted FSE (FOV= 40x40 mm, matrix=256x238 or 128x126, slice thickness=0.8 or 1.1 mm, TR=1193 ms, TE= 11 ms). The entire workflow of our study is given in Fig. 1. The k-space data were randomly under-sampled using a uniform distribution for a total reduction factor (R) of 2.2 while preserving 32 points at the center of k-space. Each slice was reconstructed using the CS reconstruction.10 To quantitatively evaluate the performance of CS-MRI, the mean squared error (MSE) and structural similarity index measure (SSIM) were used. Subsequently, a Wistar rat brain atlas was registered to the images using the Elastix module on 3D Slicer Software, and segmentations of brain regions were obtained.11–13 Afterward, the images were z-score normalized to minimize the effect of signal variation between scans, and resampled to the same isometric resolution (0.55×0.55×0.55 mm). Eighty-six radiomics features, including first-order statistics and texture, were extracted from the somatosensory area of rat brain using PyRadiomics.14 Paired Wilcoxon signed-rank test was used to determine the differences in the radiomics features obtained from the original and CS images, and P<0.05 was considered as statistically significant. The robustness of radiomics features to CS acceleration was assessed by intraclass correlation coefficients (ICC) with confidence intervals (CI).Results
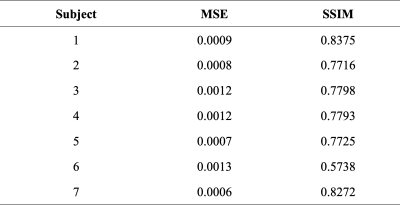
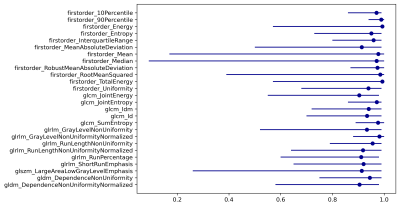
Table 1 shows MSE and SSIM values of CS reconstructed images. CS reconstruction resulted in low MSE and acceptable SSIM values. Paired Wilcoxon signed-rank analysis showed that CS images presented marked disparities compared to the original images with significant differences for up to 22 of the 86 radiomics parameters (25.58%, P<0.05 for all). The robustness of features was excellent for 26/86 (30.23%) radiomic features (ICC>0.9, Fig. 2). Additionally, 42/86 (48.84%) radiomics features displayed good robustness (ICC>0.8). However, 17 radiomics features were found to have an ICC value of less than 0.5, which were considered as not robust.Discussion
In this study, we evaluated the robustness of intensity-based radiomics features extracted from preclinical 7T MRI scanner to CS image reconstruction. Our results indicated that the majority of radiomics features didn’t significantly differ between the accelerated and original images. While 48.84% of radiomics showed good robustness with higher ICC values (ICC>0.8), 19.76% of them had an ICC value lower than 0.5. While radiomics aim to provide quantitative information of standard medical images that may be imperceptible to the human eye, they are susceptible to inhomogeneities in imaging parameters across the cohort.15 Another possible adverse influence is low SNR of preclinical images, which is more often the case compared to the clinical MRI, owing to the smaller voxel sizes.16Conclusion
Our results suggest that the radiomics features could be affected by compressed sensing acceleration and reconstruction at 7T.Acknowledgements
No acknowledgement found.References
1. Cesarovic N, Nicholls F, Rettich A, et al. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim. 2010;44(4):329-336. doi:10.1258/la.2010.009085
2. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-1195. doi:10.1002/mrm.21391
3. Zong X, Lee J, John Poplawsky A, Kim SG, Ye JC. Compressed sensing fMRI using gradient-recalled echo and EPI sequences. NeuroImage. 2014;92:312-321. doi:10.1016/j.neuroimage.2014.01.045
4. Smith DavidS, Li X, Gambrell JV, et al. Robustness of Quantitative Compressive Sensing MRI: The Effect of Random Undersampling Patterns on Derived Parameters for DCE- and DSC-MRI. IEEE Trans Med Imaging. 2012;31(2):504-511. doi:10.1109/TMI.2011.2172216
5. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278(2):563-577. doi:10.1148/radiol.2015151169
6. Zhou M, Scott J, Chaudhury B, et al. Radiomics in Brain Tumor: Image Assessment, Quantitative Feature Descriptors, and Machine-Learning Approaches. Am J Neuroradiol. 2018;39(2):208-216. doi:10.3174/ajnr.A5391
7. Scalco E, Belfatto A, Mastropietro A, et al. T2w‐MRI signal normalization affects radiomics features reproducibility. Med Phys. 2020;47(4):1680-1691. doi:10.1002/mp.14038
8. Haniff NSM, Abdul Karim MK, Osman NH, Saripan MI, Che Isa IN, Ibahim MJ. Stability and Reproducibility of Radiomic Features Based Various Segmentation Technique on MR Images of Hepatocellular Carcinoma (HCC). Diagnostics. 2021;11(9):1573. doi:10.3390/diagnostics11091573
9. Yang F, Dogan N, Stoyanova R, Ford JC. Evaluation of radiomic texture feature error due to MRI acquisition and reconstruction: A simulation study utilizing ground truth. Phys Med. 2018;50:26-36. doi:10.1016/j.ejmp.2018.05.017
10. Blumenthal M, Holme C, Roeloffs V, et al. mrirecon/bart: version 0.8.00. Published online September 24, 2022. doi:10.5281/ZENODO.592960
11. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323-1341. doi:10.1016/j.mri.2012.05.001
12. Klein S, Staring M, Murphy K, Viergever MA, Pluim J. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans Med Imaging. 2010;29(1):196-205. doi:10.1109/TMI.2009.2035616
13. Johnson GA, Laoprasert R, Anderson RJ, et al. A multicontrast MR atlas of the Wistar rat brain. NeuroImage. 2021;242:118470. doi:10.1016/j.neuroimage.2021.118470
14. van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21):e104-e107. doi:10.1158/0008-5472.CAN-17-0339
15. Piazzese C, Foley K, Whybra P, Hurt C, Crosby T, Spezi E. Discovery of stable and prognostic CT-based radiomic features independent of contrast administration and dimensionality in oesophageal cancer. Hsieh JCH, ed. PLOS ONE. 2019;14(11):e0225550. doi:10.1371/journal.pone.0225550
16. Park BW, Kim JK, Heo C, Park KJ. Reliability of CT radiomic features reflecting tumour heterogeneity according to image quality and image processing parameters. Sci Rep. 2020;10(1):3852. doi:10.1038/s41598-020-60868-9