2890
A radiomic approach to the diagnosis of femoroacetabular impingement
Eros Montin1,2, Richard Kijowski3, and Riccardo Lattanzi1,2,4
1Center for Advanced Imaging Innovation and Research (CAI2R) Department of Radiology, Radiology Department, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 3Department of radiology, New York University Grossman School of Medicine, New York, NY, United States, New York, NY, United States, 4Vilcek Institute of Graduate Biomedical Sciences, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States
1Center for Advanced Imaging Innovation and Research (CAI2R) Department of Radiology, Radiology Department, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 3Department of radiology, New York University Grossman School of Medicine, New York, NY, United States, New York, NY, United States, 4Vilcek Institute of Graduate Biomedical Sciences, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States
Synopsis
Keywords: Radiomics, MSK
The results of this study showed that radiomic can automatically distinguish a healthy joint from one with impingement using water-only Dixon MRI. To our knowledge, this is the first application of radiomic for FAI diagnosis. Our radiomic analysis achieved an accuracy greater than 97%, which is higher than the 90% accuracy for detecting FAI reported for standard diagnostic tests (90%). Combining our proposed radiomic analysis with methods for automated joint segmentation could be used to rapidly identify patients with FAI, avoiding time-consuming radiological measurements of bone morphology.Introduction
Femoroacetabular Impingement (FAI) is a common cause of hip pain in young adults, which is characterized by impingement of the femoral head-neck junction against the acetabular rim due to underlying abnormalities in bone morphology [1,2]. FAI is typically diagnosed using MRI, which can assess bone morphology through a wide variety of standardized measurements and identify underlying cartilage and labrum injuries [3]. Radiomic can predict and character pathologies by extracting quantitative features from diagnostic images [5-7]. This study was performed to assess, for the first time [8], the feasibility of using radiomic to detect FAI from MRI.Material and Methods
Seventeen patients (13F/4M, 37.1±5.7yrs) with suspected FAI underwent an MRI of the hip before arthroscopic hip surgery. Three patients had a follow-up MRI after one year. The MRI included a 3D Dixon water-only sequence of the pelvis [9,10] (Figure 1).A musculoskeletal radiologist delineated Regions Of Interest (ROIs) for the femur and acetabulum of both hips on each image slice. Left and right hip ROIs were subdivided into healthy joints (HJ) and joints with impingement (IJ) according to the surgical report (Figure 1) The IJ on follow-up MRI was excluded, which led to a total of 37 hip datasets.MR images and ROIs were rototranslated 60 times per dataset (Figure 2) to obtain a total of 2220 datasets (dAug). Rotations were randomly selected from a uniform distribution of ±5 degrees for the first two Euler’s angles (left/right axes and anterior/posterior) and ±15 degrees for the inferior/superior one. Translations were between ±5mm. To decouple the algorithms’ performance from image resolution, the datasets were resampled at 1mm isotropic and 0.4x0.4x1.2mm.For every 2220 datasets, 182 features were extracted: 91 for the femur and 91 for the acetabulum [4,5,10]. The 91 features were classified into three main classes: (i) intensity-based features (FOS), (ii) texture features, and (iii) shape and size (SS). A complete list of all features is shown in Table1. The histogram settings for all the feature classes were set to 32 bins min/max equal to 0/200. The radius of the gray-level co-occurrence matrices (GLCM) and gray-level run-length matrices (GLRLM) was set to one voxel.Two of the 17 patients (240 augmented datasets) were randomly selected for model validation, leaving a total of 1980 datasets for model training.24 subsets were defined with a variable number of features, divided by type (Table 2) and region (femur, acetabulum). For each subset, a univariate ANOVA F-value analysis was applied to find the 5 most correlated features based on p-value among those included. This yielded 24 additional subsets (f-contrasted) with 5 features each, for a total of 48 subsets (Table 2). For each subset, a K-nearest neighbor model (k=3) was trained 100 times, each time by randomly subdividing training and testing sets with a ratio of 75/25%. The inputs of each model were the values of the radiomic features in the subset, and the outputs were the labels HJ and IJ.The trained model with the highest prediction accuracy was selected as the final model for the particular subset of features and was evaluated against the validation dataset to assess its performance in identifying the IJ. To avoid biases in the feature values, the data were z-scored for each training repetition. The whole process resulted in one trained model for each of the 48 subsets of features.Results and Discussions
Results are summarized in Table 2. The best classification model in terms of prediction max accuracy and lower number of features was the GLCM f-contrasted subset (Accuracy: 0.972). Among its five features, GLCM Max Probability* (P<0.05) and both femur and acetabulum GLCM Correlation**(P<0.01), had a statistically significantly higher value for HJ than IJ. This suggests that the gray image values in the image are more homogeneously distributed in the HJ, which was confirmed by the fact that the acetabulum GLCM Inverse Variance and the femur GLCM Energy** subsets had higher values in the IJ. In fact, the features in these subsets had high values when the elements of the GLCM matrix were sparse [11], which implies texture heterogeneity in the images. This high heterogeneity of the water-only Dixon images for the IJ suggests a complex pattern of water distribution within the femur and acetabulum, which could be due to stress-related inflammation resulting from impingement. While the results reported above were obtained using both the segmented femur and acetabulum, the performance was higher using features associated only with the femur (Accuracy: 0.977). This will be further investigated to assess whether FAI can be accurately detected using only the femur ROI, which can be reliably segmented with deep learning [9].Conclusions
Our study showed that radiomic can automatically distinguish IJ from HJ using water-only Dixon MRI. To our knowledge, this is the first application of radiomic for FAI diagnosis. Our radiomic analysis achieved an accuracy greater than 97%, which is higher than the 90% accuracy for detecting FAI reported for standard diagnostic tests (90%) [12]. Combining our proposed radiomic analysis with methods for automated joint segmentation [9] could be used to rapidly identify patients with FAI, avoiding time-consuming radiological measurements of bone morphology.Acknowledgements
his work was supported by NIH R01 AR070297 and performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), an NIBIB National Center for Biomedical Imaging and Bioengineering (NIH P41 EB017183).References
- Griffin, D. R., Dickenson, E. J., O’Donnell, J., Agricola, R., Awan, T., Beck, M., Clohisy, J. C., Dijkstra, H. P., Falvey, E., Gimpel, M., Hinman, R. S., Hölmich, P., Kassarjian, A., Martin, H. D., Martin, R., Mather, R. C., Philippon, M. J., Reiman, M. P., Takla, A., … Bennell, K. L. (2016). The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): An international consensus statement. British Journal of Sports Medicine, 50(19), 1169–1176. https://doi.org/10.1136/bjsports-2016-096743
- Naili, J. E., Stålman, A., Valentin, A., Skorpil, M., & Weidenhielm, L. (2021). Hip joint range of motion is restricted by pain rather than mechanical impingement in individuals with femoroacetabular impingement syndrome. Archives of Orthopaedic and Trauma Surgery. https://doi.org/10.1007/s00402-021-04185-4
- Saied, A. M., Redant, C., El-Batouty, M., El-Lakkany, M. R., El-Adl, W. A., Anthonissen, J., Verdonk, R., & Audenaert, E. A. (2017). Accuracy of magnetic resonance studies in the detection of chondral and labral lesions in femoroacetabular impingement: systematic review and meta-analysis. BMC Musculoskeletal Disorders, 18(1). https://doi.org/10.1186/s12891-017-1443-2
- Bologna, M., Corino, V. D. A., Montin, E., Messina, A., Calareso, G., Greco, F. G., Sdao, S., & Mainardi, L. T. (2018). Assessment of Stability and Discrimination Capacity of Radiomic Features on Apparent Diffusion Coefficient Images. Journal of Digital Imaging. https://doi.org/10.1007/s10278-018-0092-9
- Corino, V. D. A., Montin, E., Messina, A., Casali, P. G., Gronchi, A., Marchianò, A., & Mainardi, L. T. (2018). Radiomic analysis of soft tissue sarcomas can distinguish intermediate from high-grade lesions. Journal of Magnetic Resonance Imaging, 4(3), 829–840. https://doi.org/10.1002/jmri.25791
- Bologna, M., Calareso, G., Resteghini, C., Sdao, S., Montin, E., Corino, V., Mainardi, L., Licitra, L., & Bossi, P. (2020). Relevance of apparent diffusion coefficient features for a radiomics-based prediction of response to induction chemotherapy in sinonasal cancer. <i>NMR in Biomedicine</i>, <i>March 2019</i>, 1–12. https://doi.org/10.1002/nbm.4265
- Gitto, S., Cuocolo, R., Albano, D., Morelli, F., Pescatori, L. C., Messina, C., Imbriaco, M., & Sconfienza, L. M. (2021). CT and MRI radiomics of bone and soft-tissue sarcomas: a systematic review of reproducibility and validation strategies. <i>Insights into Imaging</i>, <i>12</i>(1), 68. https://doi.org/10.1186/s13244-021-01008-3
- Mascarenhas, V.V., Caetano, A., Dantas, P. et al. Advances in FAI Imaging: a Focused Review. Curr Rev Musculoskelet Med 13, 622–640 (2020). https://doi.org/10.1007/s12178-020-09663-7
- Montin, E., Deniz, C. M., Rodrigues, T. C., Gyftopoulos, S., Kijowski, R., ; Lattanzi, R. (2022). Automatic segmentation of the hip bony structures on 3D Dixon MRI datasets using transfer learning from a neural network developed for the shoulder. 30th Scientific Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM) London (UK), 07-12 May 2022, 1412.
- Cantarelli Rodrigues, T., Deniz, C. M., Alaia, E. F., Gorelik, N., Babb, J. S., Dublin, J.,; Gyftopoulos, S. (2020). Three-dimensional MRI Bone Models of the Glenohumeral Joint Using Deep Learning: Evaluation of Normal Anatomy and Glenoid Bone Loss. <i>Radiology: Artificial Intelligence</i>, <i>2</i>(5), e190116.
- Reiman, M. P., Goode, A. P., Cook, C. E., Hölmich, P., & Thorborg, K. (2015). Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: A systematic review with meta-analysis. In <i>British Journal of Sports Medicine</i> (Vol. 49, Issue 12, p. 811). BMJ Publishing Group. https://doi.org/10.1136/bjsports-2014-094302
- Dhruv B, Mittal N, Modi M. Study of Haralick's and GLCM texture analysis on 3D medical images. Int J Neurosci. 2019 Apr;129(4):350-362. doi: 10.1080/00207454.2018.1536052. Epub 2018 Dec 20. PMID: 30311815.
Figures
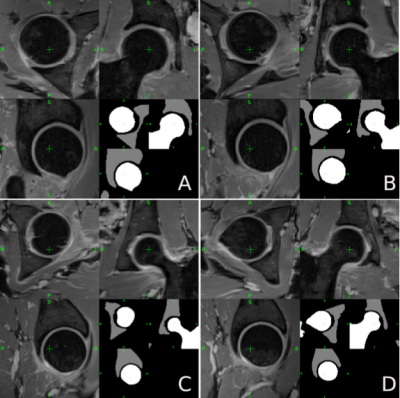
Two Examples of healthy (A, C) and impinged (C, D) hip joints on two patients. For each image axial, coronal, and sagittal view. Images were acquired with a FOV=320x320mm2, 320x320 matrix size, 1mm slice thickness without gap, centered on the patient's pelvis. In the lower part of each sub-image, an example of the ROI drawn by (RR) is shown, in particular in white the femur ROI and in gray the acetabulum one.
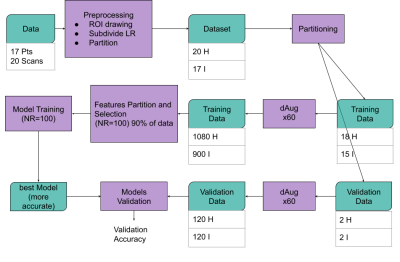
Schematic representation of the workflow of the study. Data are pre-processed and a total of 33 datasets (18 Healthy and 15 Impinged) (ROIs and images) are used for the model training phase while 4 are used for validation. Training and validation dataset numerosity was augmented using a factor of 60.48 subsets of features created by randomly taking 75% of training data. For each subset of features, a KNN ML process was repeated 100 times and the most accurate ones were selected as the final model. Finally, the [performance of the best model for each subset was accessed on the validation data
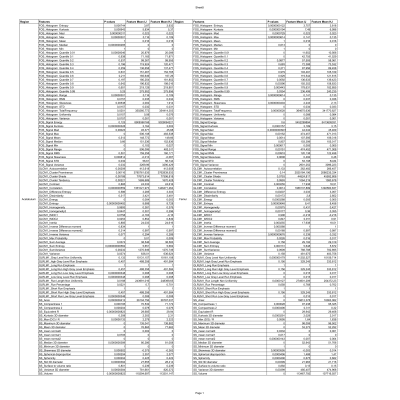
the list of the radiomic features Used in this study.For each feature, the p values of the Wilcoxon rank-sum test are reported along with the mean values for the impinged and healthy distribution of the values of the feature.
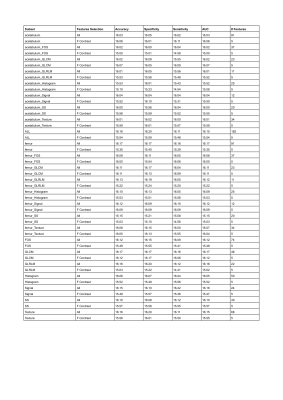
Reports the accuracy, specificity, sensitivity, and AUC for all the 48 subsets of features. The complete list of features contained in each subset can be seen in Table 1
DOI: https://doi.org/10.58530/2023/2890