2889
Rado – A Cloud-Based Toolbox for Radiomics Analysis
Eros Montin1,2 and Riccardo Lattanzi1,2,3
1Center for Advanced Imaging Innovation and Research (CAI2R) Department of Radiology, Radiology Department, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 3Vilcek Institute of Graduate Biomedical Sciences, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States
1Center for Advanced Imaging Innovation and Research (CAI2R) Department of Radiology, Radiology Department, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States, 3Vilcek Institute of Graduate Biomedical Sciences, New York University Grossman School of Medicine, New York, New York, USA, New York, NY, United States
Synopsis
Keywords: Radiomics, Software Tools
Rado is a web-based application designed to fully automatically perform a radiomic analysis. Rado allows users to extract features from medical images, apply feature selections, and mine the dataset using the most common classifiers and regressors. It also offers the option to augment the dataset by means of rigid transformations.The current version of Rado allows users to interact with the dataset by means of restful APIs or using a standardized web GUI. The application will be distributed via the Cloud MR portal, which allows running the feature extraction on sparse servers as well as on local computers.Introduction
Radiomics is an emerging field in quantitative imaging for cancer diagnostic, staging, and therapy evaluation [1,2,3]. By extracting features that quantify the spatial distribution of signal intensities and pixel textural interrelationships in an image and evaluate their correlation with subject outcomes, radiomics can noninvasively assess lesions phenotypes, therapy treatment, and cancer staging [3]. Various research groups have developed in-house feature extractors or data mining applications [4], but a certain level of programming and software engineering skills is still required to perform a complete radiomic analysis, which has limited the adoption of radiomics [4].Thus, we present Rado, an open-source, cloud-based web application that allows experienced radiomic operators, as well as less trained ones, to conduct a full radiomic analysis in a standard way using state-of-the-art software for feature extraction. Rado transforms images into a new domain, where artificial intelligence algorithms can find recursive patterns, correlation, and causality between extracted features and clinical outcomes. Rado will be incorporated within Cloud MR, a portal that provides access to MRI simulation and image processing software through a web-based graphical user interface (GUI). The modular software architecture of Cloud MR enables users to efficiently run Rado via Docker containers, either on local computers or using cloud computing services (Figure 1)Software Architecture and Features
Rado has been developed within the Cloud MR framework (http://cloudmrhub.com, in beta testing, not yet publicly available), which relies on a standardized web-based GUI [6,7] and a computing unit that executes the features extraction via Docker containers. The ‘Set Up’ tab in the GUI (Figure 2) allows users to upload images and ROIs (nifti, nrrd, and mha files). From one of the panels, users select the radiomic features they want to extract among the shape and size features, first-order statistics (FOS [8]) and the textural features (Gray level cooccurrence and long-run matrix)[8]. In the subsequent panel, users can customize the feature extraction by selecting the options related to the histogram definition (min, max, number of bins) and the radius of the textural analysis. From the classification panel, users can select the most common classifiers, such as K Nearest neighbors, support vector machines, and customize a Neural network, as well as select the training/validation set ratio. The same customization can be sent to the backend unit of Rado from a Matlab or python script by means of restful APIs. Our set of APIs can be useful when the feature extraction is performed, for example, inside an optimization loop. The ‘Results’ tab of the GUI shows the status of the scheduled extractions and allows users to download the results of the extraction as JSON files, see the heatmap of their data (Figure 3) as well as a radar plot of the most correlated features (Figure 4).The backend of Rado relies on the three layers architecture as all Cloud MR’s web apps. Similar to the human motor system (Figure 5), the upper level of the “brainstem” manages communications with the basic computational units and the GUI using a flask image. The “spinal node” passes the commands and options to the operative level and the operative level, “the muscle”, performs the feature extraction, data augmentation, and mining by means of a dockerize version of the software that we used in our previous radiomics works [1-3]. When the task is completed, the muscle starts the chain of requests that eventually propagates the results back to the brainstem via the spinal node. This modularity allows users to integrate Rado input and output with other web application computing units.On top of this 3-layers architecture, our cortex service allows users to sync data and task results, through the cerebrum part of Figure 5.Discussion and Conclusion
We introduced Rado, a web-accessible application for radiomic analysis. By containerizing the most common radiomic feature extractors, data augmentation, and machine learning, Rado allows users, even the less trained ones, to customize an entire radiomic analysis by means of a simple GUI.By exploiting the modularity of the Cloud MR architecture, we plan to develop an enriched version that integrates other features extractors, like pyradiomic [11], image anonymizers, like Pydeface and Maskface [9,10], then distributed as a new basic unit for Rado and for the Cloud MR community. We plan to integrate Rado with our previously presented applications, for example, MR Optimum [6], our SNR evaluator, and CAMRIE [7], our MRI emulator. Among them, the integration of CAMRIE [7] inside Rado will allow users to create syntetic-generated images to eventually decrease the number of scans needed for the training of the radiomic models. This could potentially diminish the number of healthy subjects’ scans in a radiomic study, focusing the resources on real patient data with a potential reduction of the cost of research.The current version of Rado allows users to evaluate binary classifications but we are planning to add the most common regression analysis in the next versions. Three abstracts submitted for ISMRM 23 used Rado for their radiomic analysisAcknowledgements
This work was supported by NIH R01 AR070297 and performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), an NIBIB National Center for Biomedical Imaging and Bioengineering (NIH P41 EB017183).References
- Bologna, M., Calareso, G., Resteghini, C., Sdao, S., Montin, E., Corino, V.D.A., Mainardi, L. T., Licitra, L., Bossi, P., , “Relevance of apparent diffusion coefficient features for a radiomics-based prediction of response to induction chemotherapy in sinonasal cancer,” NMR Biomed., no. March 2019, pp. 1–12, 2020.
- Bologna, M., Corino, V. D.A., Montin, E., Messina, A., Calareso, G., Greco, F. G., Sdao, S., Mainardi, L. T. “Assessment of Stability and Discrimination Capacity of Radiomic Features on Apparent Diffusion Coefficient Images,” Journal of Digital Imaging, 2018.
- Corino, V. D.A., Montin, E., Messina, A., Casali, P G., Gronchi, A., Marchianò, A., Mainardi, L. T., “Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions,” J. Magn. Reson. Imaging, vol. 47, no. 3, pp. 829–840, 2018.
- Yuan, R., Shi, S., Chen, J. et al. Radiomics in RayPlus: a Web-Based Tool for Texture Analysis in Medical Images. J Digit Imaging 32, 269–275 (2019). https://doi.org/10.1007/s10278-018-0128-1
- van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017 Nov 1;77(21):e104-e107. doi: 10.1158/0008-5472.CAN-17-0339. PMID: 29092951; PMCID: PMC5672828.
- E. Montin, R. Wiggins, K. T. Block, and R. Lattanzi, “MR Optimum – A web-based application for signal-to-noise ratio evaluation” in ISMRM 27th annual meeting and exhibition 11-16 May 2019, Program number: 4617
- E. Montin, G. Carluccio, C. Collins, R. Lattanzi “CAMRIE - Cloud-Accessible MRI Emulator” in ISMRM 28th annual meeting and exhibition 08 -14 Aug 2020, Program number: 1037
- Aerts, H., Velazquez, E., Leijenaar, R. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5, 4006 (2014). https://doi.org/10.1038/ncomms5006
- Gulban, O.F., Nielson, D., Poldrack, R., Lee, J., Gorgolewski, C., Vanessasaurus, Ghosh, S., 2019. poldracklab/pydeface: v2.0.0. Zenodo. https://doi.org/10.5281/zenodo.3524401
- Milchenko, M., Marcus, D., 2013. Obscuring Surface Anatomy in Volumetric Imaging Data. Neuroinformatics 11, 65–75. https://doi.org/10.1007/s12021-012-9160-3 [11] Griethuysen, J. J. M., Fedorov, A., Parmar, C., Hosny, A., Aucoin, N., Narayan, V., Beets-Tan, R. G. H., Fillon-Robin, J. C., Pieper, S., Aerts, H. J. W. L. (2017). Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Research, 77(21), e104–e107. `https://doi.org/10.1158/0008-5472.CAN-17-0339 <https://doi.org/10.1158/0008-5472.CAN-17-0339>`_
Figures
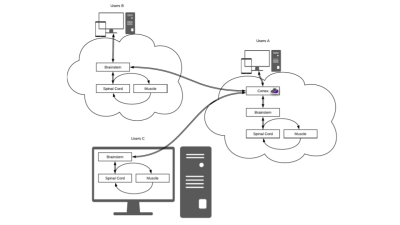
User A requests simulations from the web GUI and runs them on a dedicated Cloud MR AWS account. User B runs the simulations from the web GUI using her/his own cloud computing account. User C runs the simulations on a local computer. Users B and C can synchronize the results on Cloud MR using restful API’s. All types of users can display results on the web GUI or download them in standard formats (e.g., mat or json).
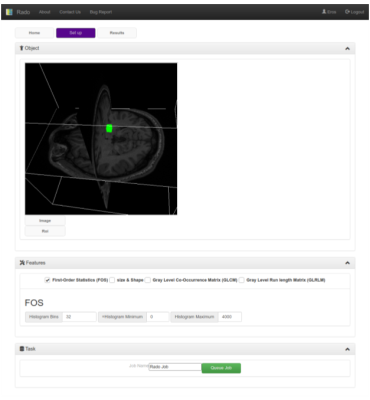
The Web-GUI is implemented in HTML and uses several javascript libraries (React js, three.js, and ami.js). In the Set up tab, users can customize the feature extraction by uploading images, and ROIs and changing the features configurations like histogram bins and ranges. In the Home tab users can manage their data (delete or download) In The results tab (not shown) users can download the results of the extraction as JSON files
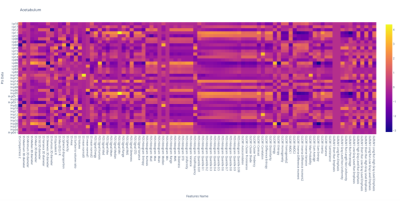
An example of the heatmap obtained by the feature values extracted with Rado. Each row of the heatmap represents a patient of the study while the columns report the feature value throughout the population. For example, the FOS mean column reports the mean values of the grayscale image values in the ROI of all patients.
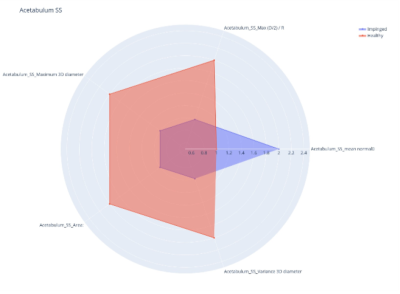
An example of the radar plot output of Rado, which allows users to visualize the values of the features extracted from the patients in the study. The example in this figure reports the values of a subset of the shape and size features of a 2-label classification radiomic analysis for the diagnosis of pathologic hip joints (impinged, healthy).In this example, five features are reported and, for each of them, the mean of the two classes is reported.
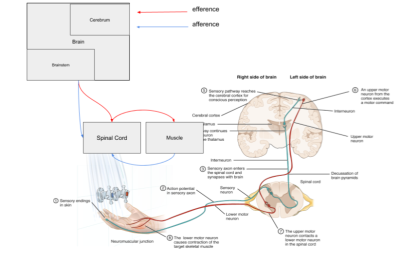
Rado’s internal architecture mimics the human motor system by using restful APIs and docker images. Users can send task requests through a set of APIs that can be customized by the WebGUI or from any tool able to send HTML requests. The request is received by the brainstem, a python backend based on flask, that sends the request to the spinal node, which is a second python backend that inserts the task in the muscle’s queue of jobs. This layer is composed of Celery and Redis, a locally cached database of tasks.
DOI: https://doi.org/10.58530/2023/2889