2887
The value of regional radiomics score based on postoperative conventional MRI in evaluation of glioma recurrence1Department of MR, The First Affiliated Hospital of Xinxiang Medical University, Weihui, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
Keywords: Radiomics, Radiomics
Early diagnosis of postoperative glioma recurrence is difficult. But emerging measurements of radiomics could provide a powerful tool for this dilemma. We used the least absolute shrinkage and selection operator to select features and generate radiomics scores based on multiple modalities of conventional MRI to discriminate recurrence from treatment-related effects. We found that tumor recurrence could be independently identified by features from both the postoperative enhanced region and edematous region with a best performance of the combined one.Introduction
Glioma is the most common brain tumor with characteristics of high recurrence and mortality rate1. Unfortunately, many patients will recur in a short time or have poor prognoses after postoperative treatment. In addition, inflammatory processes after chemoradiation that simulate the signs of tumor recurrence are often mistaken for tumor progression, which often affects the flowing treatment scheme. Therefore, early recognition of recurrence is essential for diminishing malignant transformation of recurrent and extending survival time. Conventional MRI is widely applied in clinical works, yet confined by radiologist’s experience and affected by subjective judgments. Radiomics are used to extract quantitative features from radiographic images and to deeply mine tissue information. This method has been proven to be a useful tool in glioma grading, subtype classification, and tumor proliferation prediction2,3. Here, we aim to use the least absolute shrinkage and selection operator (LASSO) algorithm to construct a machine learning model based on rad-score for clinical diagnosis of glioma recurrence.Methods
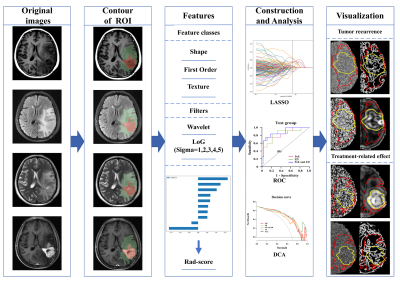
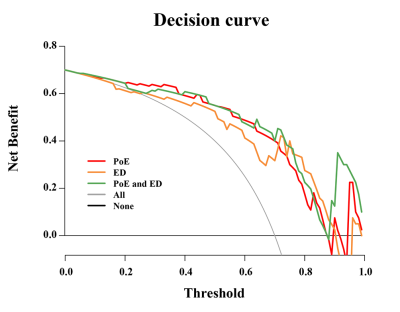
This retrospective study was approved by the ethics committee and consent waived. First, 131 patients were included in the primary cohort, according the RANO criteria, 72 were considered to have tumor recurrence and 59 for treatment-related effects. The patients were randomized in a 7:3 ratio into a training group (n=90) and a test group (n=41). All features were extracted from four routine sequences (T1-weighted imaging, contrast enhanced T1-weighted imaging, T2-weighted imaging, T2-fluid attenuation inversion recovery) and two regions of interest, i.e., peritumor edema (ED) region and the postoperative enhancement (PoE) region. The intraclass correlation coefficient was used to select stable features, and spearman's rank correlation coefficients were obtained to eliminate redundant features. The LASSO algorithm with penalty parameter tuned by 10-fold cross-validation was employed to select glioma recurrence-related factors and to calculate their weighted coefficients. According to different features of weighted coefficients from multiple sequences, we select five features with high weighted coefficients from the postoperative enhancement (PoE) region and edema (ED) region respectively. For the whole region combining PoE region and ED region, we selected the top ten high weighted coefficients features. Then rad-score was computed for the selected recurrence-related radiomics features and the corresponding weights. Furthermore, in training and test cohorts, the rad-score was used to construct three models based on PoE region, ED region, and the whole region. Decision curve analysis (DCA) was performed to quantify the clinical utility of the three models (Fig.1).Results
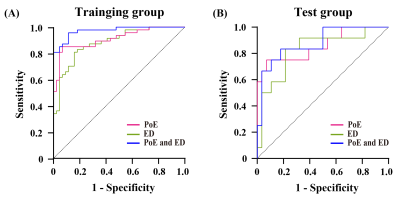
Finally, the selected features included 3 first-order features and 7 second-order features. Among these features, 5 features derived from wavelet filters and 4 features originated from Log filters. In the training group, the AUC of the PoE and ED regions was 0.912 (95 % CI: 0.852−0.972) and 0.882 (95 % CI: 0.815−0.950), respectively (Fig.2A). In the test cohort, the AUC of the PoE and ED regions was 0.860 (95 % CI: 0.721−0.999) and 0.827 (95 % CI: 0.679−0.975), respectively (Fig.2B). Among the three models, the whole region model showed the best performance in predicting glioma recurrence with the AUC of 0.973 (95 % CI: 0.946−1.000) and 0.878 (95 % CI: 0.759−0.997) in the training group and test group, respectively. The DCA illustrated that, all the three models are beneficial for diagnosing glioma recurrence when the threshold probability was over 0.2, and the performance of multiregional model was better than the ED models in most situations Figure 3.Discussion
Among the PoE, the ED model, and the combined whole-region model, the whole region model has the best performance for differentiating recurrence from treatment-related effects. Selected features were mostly come from T2WI and CE-T1WI. The T2WI demonstrates hyperintensity due to increase in water content and is a useful for revealing the biological information of peritumoral edema. In addition, we confirmed that the ED region contains valuable information for diagnosis, which was consistent with previous reports that tumor cells could be residual in peritumor edema4,5. Glioma can destruct the brain-blood barrier, enhance endothelial cell permeability, and cause rapid capillary proliferation. Consequently, CE-T1WI showed higher signal intensity which can better imply tissue malignancy. On the other hand, the previous study suggested that Wavelet-based features had a strong ability to dig out the information of tumor heterogeneity at different dimensionalities. In this study, this kind of features similarly play a key role in distinguishing recurrence.Conclusion
Regional rad-score based on conventional images is a considerable tool in identifying postoperative glioma recurrence from treatment-related effects. Besides, combining features from the postoperative enhancement region and the edema region can improve the differentiation performance.Acknowledgements
No acknowledgement found.References
1.Sharma HS, Muresanu DF, Castellani RJ, et al. Pathophysiology of blood-brain barrier in brain tumor. Novel therapeutic advances using nanomedicine. Int Rev Neurobiol. 2020; 151: 1-66.
2.Gao XY, Wang YD, Wu SM, et al. Differentiation of Treatment-Related Effects from Glioma Recurrence Using Machine Learning Classifiers Based Upon Pre-and Post-Contrast T1WI and T2 FLAIR Subtraction Features: A Two-Center Study. Cancer Manag Res. 2020; 12: 3191-3201.
3.Zha H, Zong M, Liu X, et al. Preoperative ultrasound-based radiomics score can improve the accuracy of the Memorial Sloan Kettering Cancer Center nomogram for predicting sentinel lymph node metastasis in breast cancer. Eur J Radiol. 2021; 135: 109512.
4.Barajas RJ, Phillips JJ, Parvataneni R, et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol. 2012; 14(7): 942-954.
5.Meng X, Xia W, Xie P, et al. Preoperative radiomic signature based on multiparametric magnetic resonance imaging for noninvasive evaluation of biological characteristics in rectal cancer. Eur Radiol. 2019; 29(6): 3200-3209.
Figures