2883
Reproducibility of radiomics features between MRI-derived synthetic-CT and true CT in prostate MR-guided radiotherapy1Research Department, Hong Kong Sanatorium and Hospital, Hong Kong, China, 2Medical Physics Department, Hong Kong Sanatorium and Hospital, Hong Kong, China
Synopsis
Keywords: Radiomics, Prostate, MRCAT, synthetic-CT, reproducibility
MR-guided radiotherapy and radiomics have gained considerable attention. Synthetic-CT (sCT) derived from MRI has been adopted to facilitate MRI-only radiotherapy planning. Researches have evaluated the sCT both qualitatively and quantitatively. However, few studies have looked into the potential that sCT offers at radiomics level. We hypothesize that sCT generated from MR can faithfully reproduce radiomics features compared to those extracted from true planning-CT. We aim to investigate the reproducibility of radiomics features derived from a commercially available sCT generation (MRCAT) acquired on a 1.5T MR-simulator to those obtained from a CT simulation scan in a cohort of prostate cancer patients.Introduction
MR-guided radiotherapy (MRgRT) and radiomics have gained considerable attention in prostate cancer management. Currently, most radiomics studies were based on multi-parametric MRI. The lack of electron density information in MRI poses a major challenge in radiotherapy treatment planning (RTP), so synthetic-CT (sCT) derived from MRI has been developed and increasingly adopted to facilitate MRI-only RTP [1]-[5]. Researches have extensively evaluated the sCT both qualitatively in terms of image quality and quantitatively in terms of Hounsfield Unit (HU) per electron density calibration [6]-[10]. However, few studies have looked into the potential value that sCT offers at the radiomics feature level. We hypothesize that sCT generated from MR can faithfully reproduce radiomics features compared to those extracted from true planning-CT images. We aim to investigate the reproducibility of radiomics features extracted from a commercially available sCT generation with continuous HU estimation, MR for Calculating ATtenuation (MRCAT) pelvis based on 3D-GRE Dixon fat-water imaging [11]-[13], acquired on a 1.5T MR-simulator, to those acquired from CT simulation scan, in a cohort of prostate cancer patients.Methods
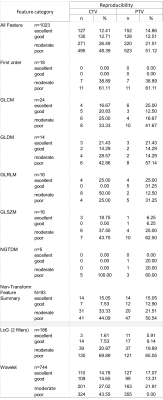
This retrospective study was approved by the Hospital research ethics committee, with wavier of informed consent. Fifty prostate cancer patients undergoing prostate MRgRT were included. All included patients underwent a CT scan on a CT-sim scan (SOMATOM Confidence, Siemens Healthcare) and subsequently an MRI scan on a 1.5T MR-sim (Ingenia MR-RT, Philips Healthcare, Best, Netherlands), with a time interval of 15-60 minutes, both in the same treatment position. The imaging parameters were presented in Table I.An experienced radiation oncologist segmented the whole prostate as clinical target volume (CTV) on the planning CT. PTV was generated by the isotropic expansion of CTV by 5mm in all directions. The sCT images were then rigidly registered and resampled to have the same resolution and coordinate system to the planning-CT. Manual check was performed, focusing on the match of anatomies in the CTV to maximize the tissue-of-interest consistency. CTV and PTV were then propagated to the sCT images. Totally 1023 radiomics features, including 93 original features, and 930 transformed features using wavelet and Laplacian-of-Gaussian (LoG), were extracted in the CTV and PTV from each dataset, with the default fixed bin size of 25 in pyRadiomics.
Two-way mixed effects, consistency, single measurement ICC model was used for feature reproducibility cohorts between sCT and planning CT. ICCs were classified as excellent (ICC>0.9), good (0.9>ICC>0.75), moderate (0.75>ICC>0.5) and poor (ICC>0.5).
Statistical analyses were conducted in R v1.2. ANOVA test with Bonferroni correction was conducted to compare the feature ICCs, bias, and LoA for the synthetic- and planning CT in CTV and PTV. Chi-square test was conducted to compare the ICC classification distribution for different feature categories in different cohorts. A P-value < 0.05 indicated statistical significance.
Results
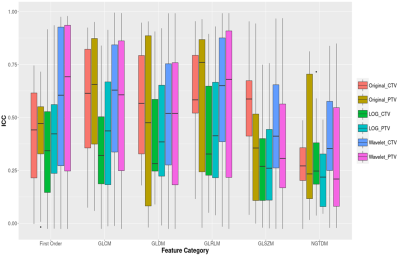
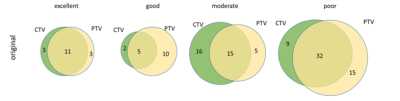
The ICC classification results were summarized in Table II. Overall, about 257 (25.1%) and 280 (28.3%) out of 1023 features showed ICCs≥0.75 in the CTV and PTV, respectively, representing excellent and good reproducibility between sCT and true CT. For the original features, there were 21 (22.58%) and 26 (27.95%) out of 93 features exhibited good reproducibility, in CTV and PTV respectively. Specifically, there were no features have ICC>0.75 in the first-order group (FO), in either CTV or PTV. NGTDM features derived from CTV were generally non-reproducible, with all 5 features have ICC<0.05. No significant differences were found between feature categories in the original features group (all p>0.05). Figure 1 illustrated the boxplot of the ICCs of features extracted from PTV and CTV grouped into different feature categories. The Venn diagram presented in Figure 2 demonstrated the overlapped original features in the four ICC classes between the CTV (in green) and PTV (in yellow).Discussion and Conclusion
To our knowledge, this is the first study to investigate synthetic radiomics feature reproducibility between the planning-CT and sCT. Our preliminary results suggested that some radiomics features derived from sCT reliably maintained their quantitative properties originated from the true CT, so held potentials for pseudo-multi-model radiomics modelling based on MRI-only original images in future MRgRT studies. But, on the other hand, those features with poor reproducibility should be abandoned in modelling or be further improved. In our study, the poor reproducibility of original features in the first-order (FO) and NGTDM groups may be partially explained by the estimation uncertainty of the HU values in sCT. The wavelet transformed domain had substantial but insignificant more features with good/excellent reproducibility in both CTV and PTV than LoG features. This could be explained in part by the fact that the LoG transform emphasizes regions of rapid intensity change, whereas algorithm that assigns the voxel value in sCT may smooth out such sharp transit. The main limitation of this study is the relatively small sample size and retrospective design. It is still unknown how the MRI acquisition parameters affect the sCT radiomics features as the imaging parameters are fixed in the MRCAT acquisition protocol. Many other MRI-derived sCT generation algorithms have been also developed but their radiomics features have not yet been investigated. Further evaluation and external validation are warranted.Acknowledgements
No acknowledgement found.References
[1]. Han X. MR-based synthetic CT generation using a deep convolutional neural network method. Med Phys 2017;44(4):1408-1419.
[2]. Emami H, Dong M, Nejad-Davarani SP, Glide-Hurst CK. Generating synthetic CTs from magnetic resonance images using generative adversarial networks. Med Phys 2018.
[3]. Lei Y, Harms J, Wang T, Liu Y, Shu HK, Jani AB, Curran WJ, Mao H, Liu T, Yang X. MRI-only based synthetic CT generation using dense cycle consistent generative adversarial networks. Med Phys 2019;46(8):3565-3581.
[4]. Liu Y, Lei Y, Wang Y, Shafai-Erfani G, Wang T, Tian S, Patel P, Jani AB, McDonald M, Curran WJ, Liu T, Zhou J, Yang X. Evaluation of a deep learning-based pelvic synthetic CT generation technique for MRI-based prostate proton treatment planning. Phys Med Biol 2019;64(20):205022.
[5]. Xiang L, Wang Q, Nie D, Zhang L, Jin X, Qiao Y, Shen D. Deep embedding convolutional neural network for synthesizing CT image from T1-Weighted MR image. Med Image Anal 2018;47:31-44.
[6]. Dinkla AM, Florkow MC, Maspero M, Savenije MHF, Zijlstra F, Doornaert PAH, van Stralen M, Philippens MEP, van den Berg CAT, Seevinck PR. Dosimetric evaluation of synthetic CT for head and neck radiotherapy generated by a patch-based three-dimensional convolutional neural network. Med Phys 2019;46(9):4095-4104.
[7]. Maspero M, Savenije MHF, Dinkla AM, Seevinck PR, Intven MPW, Jurgenliemk-Schulz IM, Kerkmeijer LGW, van den Berg CAT. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys Med Biol 2018;63(18):185001.
[8]. Arabi H, Dowling JA, Burgos N, Han X, Greer PB, Koutsouvelis N, Zaidi H. Comparative study of algorithms for synthetic CT generation from MRI: Consequences for MRI-guided radiation planning in the pelvic region. Med Phys 2018;45(11):5218-5233.
[9]. Farjam R, Tyagi N, Deasy JO, Hunt MA. Dosimetric evaluation of an atlas-based synthetic CT generation approach for MR-only radiotherapy of pelvis anatomy. J Appl Clin Med Phys 2019;20(1):101-109.
[10]. Tyagi N, Fontenla S, Zelefsky M, Chong-Ton M, Ostergren K, Shah N, Warner L, Kadbi M, Mechalakos J, Hunt M. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol 2017;12(1):119.
[11]. Tyagi N, Fontenla S, Zhang J, et al, Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys Med Biol. 2017;62:2961.
[12].Kemppainen R, Suilamo S, Tuokkola T, Lindholm P, Deppe MH, Keyril¨ainen J. Magnetic resonance-only simulation and dose calculation in external beam radiation therapy: a feasibility study for pelvic cancers. Acta Oncol. 2017;56:792–798.
[13]. Kemppainen R, Suilamo S, Ranta I, et al. Assessment of dosimetric and positioning accuracy of a magnetic resonance imaging-only solution for external beam radiotherapy of pelvic anatomy. Phys Imaging Radiat Oncol. 2019;11:1–8.
Figures