2878
Working Harder at MRI Safety, But Getting Poorer MRI Safety Results.
Tobias Gilk1,2
1Gilk Radiology Consultants, Overland Park, KS, United States, 2RADIOLOGY-Planning, Kansas City, MO, United States
1Gilk Radiology Consultants, Overland Park, KS, United States, 2RADIOLOGY-Planning, Kansas City, MO, United States
Synopsis
Keywords: Safety, Safety
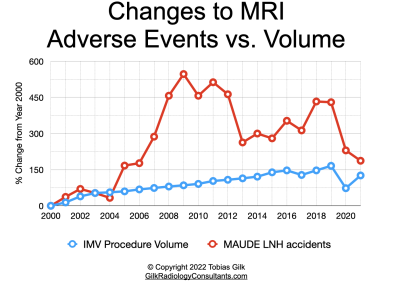
US data suggests that MRI adverse events are growing at a rate of nearly 3x the rate of MRI examinations. While MRI accidents and injuries remain infrequent, the long-term trend of significant growth in MRI adverse events highlights needs for vigilance in MRI safety.Methods
The author collected decades of data from the US FDA Medwatch program (MAUDE database), and corresponding decades of data from IMV, a radiology market research firm, to be able to compare US MRI-classified adverse event data (US FDA product code "LNH") against US MRI exam data, over time. This data comparison allows for a longitudinal analysis of changes in each MRI exam volumes performed, and MRI adverse event data reported, to be able to compare and contrast longitudinal trends between MRI adverse event data and MRI procedure volume data. To compare changes in data, for both datasets the author used the year 2000 as a reference point and measured changes in each variable over time. Linear regressions of each dataset were performed, and the slope of each dataset's linear regression line were compared over extended periods of time to identify long-term trends.Particularly because of the comparatively small number of adverse event reports, to prevent against the risk of having randomly chosen an anomalous starting-point year, comparative datasets with different initial reference years were chosen (each of the four preceding, and following years), with linear regression data comparisons for each year, with comparisons to the original (year 2000) origin to demonstrate that the chosen origin was not anomalous.
Results
The results demonstrate that the reported US MRI adverse event rate has grown, on average, almost three times the rate of MRI procedure volume in the evaluated time period.Limitations
MRI adverse events that involve other implants or medical devices are not typically classified by the US FDA as MRI adverse events, so actual adverse events are greater than those that can be readily identified in the FDA system.Acknowledgements
No acknowledgement found.References
US FDA MAUDE database.
DOI: https://doi.org/10.58530/2023/2878