2862
Association of brain connections in anterior and posterior circulation with the side of asymptomatic internal carotid stenosis and verbal memory1Department of Biomedical Imaging and Radiological Sciences, National Yang Ming Chiao Tung University, Taipei, Taiwan, 2School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan, 3Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan, 4Institute of Brain Science, National Yang Ming Chiao Tung University, Taipei, Taiwan
Synopsis
Keywords: Vessels, Ischemia
Previous studies have reported that patients with asymptomatic internal carotid stenosis (aICS) had recall verbal memory impairment. However, the underlying mechanism to elucidate the altered functional connectivity (FC) associated with the anterior and posterior circulation was not proposed. Furthermore, whether the laterality of aICS is a factor in FC alterations and verbal memory impairment requires further investigation. In this study, we reported that the left and right aICS groups showed different patterns of FC changes related to secondary language regions in anterior and posterior circulation. These FCs could be served as imaging biomarkers for recall verbal memory.Background and Purpose
In the unilateral asymptomatic internal carotid artery stenosis (aICS), perfusion of anterior circulation in lesion-side hemisphere may be reduced. To maintain brain functions, the compensation from contralateral anterior circulation and ipsilateral posterior circulation have been inspected with arterial spin labeling after aICS. [1] The altered perfusion could influence the regional brain activation and further cause deficits on recall verbal memory. [2] However, the alterations of functional connectivity (FC) caused by aICS within anterior and posterior circulation was less explored. In this study, we aimed to investigate the altered FCs and verbal memory impairment associated with the laterality of aICS in anterior- and posterior-circulation regions.Materials and Methods
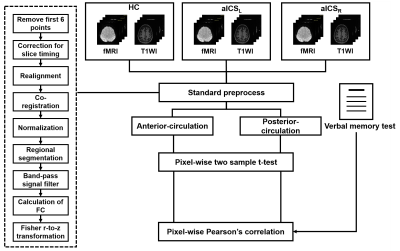
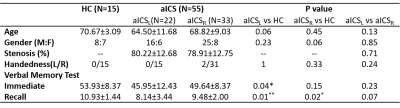
We recruited 15 healthy controls (HCs), 22 patients with left aICS (aICSL), and 33 patients with right aICS (aICSR). Patients with stenotic degree less than 50% and neurodegenerative diseases were excluded. Chinese version verbal memory test was used to evaluate the level of working (immediate) and short-term (delayed recall) verbal memory (Table 1). [3] MRI data, including 3D BRAVO T1-weighted images (TR/TE: 12.2/5.2ms; voxel size: 1×1×1mm3) and EPI BOLD fMRI (TR/TE: 3000/30ms; voxel size: 1.73×1.73×3mm3) during resting state (124 volumes) were acquired on a 3T MR scanner (GE, Discovery 750). The fMRI data were preprocessed using SPM12, including correction for slice timing, realignment, co-registration between T1WI and fMRI, spatial normalization, and spatial smoothing with a 6-mm full-width half-maximum Gaussian kernel. [4] The confounding effects of head motion and signals from white matter and cerebrospinal fluid were regressed out for the subsequent FC analysis. The targeted regions, including superior medial frontal gyrus, middle temporal pole gyrus, lingual gyrus, cerebellum 8, and vermis 8, were parceled based on the Automatic Anatomical Labeling atlas (AAL116). [5] FC between each pair of regions was estimated using Pearson’s correlation coefficient between regional BOLD signals (with a bandpass filtering between 0.01 and 0.10 Hz) followed by the Fisher’s r-to-z transform. The posterior-circulation functional remodeling was investigated in the FC matrix including middle temporal pole gyrus, cerebellum 8, and vermis 8. The anterior-circulation functional remodeling was reveled in the FC matrix including superior medial frontal gyrus and lingual gyrus. The altered FC in anterior and posterior circulation for the aICSL or aICSR groups would be identified using a two-sample t-test with the comparison to the HC group (p<0.05, FDR corrected). Correlation analysis was applied to evaluate the association of FC with the recall verbal memory function (p<0.05). The processing workflow is shown in Figure 1.Results and Discussion
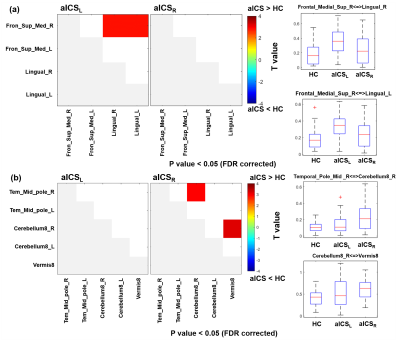
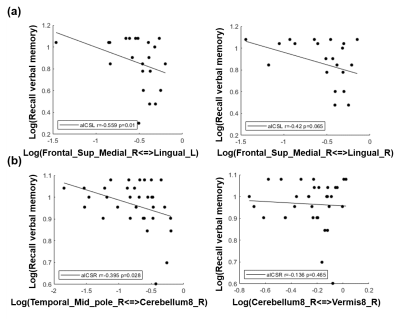
The patients with aICSL or aICSR showed significant impaired recall verbal memory compared to HCs (Table 1). No significant difference in recall verbal memory between aICSL and aICSR groups (Table 1). Figure 2 shows the significantly altered FCs in aICSL and aICSR groups compared to HC group. In the anterior circulation, aICSL group showed a higher FC between right superior medial frontal gyrus and bilateral lingual gyrus compared to HCs (Figure 2a). In the posterior circulation, aICSR group showed a higher FC of right cerebellum8 with right middle temporal pole gyrus and vermis8 compared to HCs (Figure 2b). The five target regions are involved with verbal memory. [6-9] Wang et al. and Tuo et al. also reported changed activation of superior medial frontal gyrus, lingual gyrus, middle temporal pole gyrus, cerebellum8, and Vermis8 after aICS. [10, 11] The lateralization of language-network development might be one of the reasons for the right anterior-circulation regions being activated after left aICS, and the compensation from right posterior-circulation regions being observed after right aICS. [12, 13] The FC between left broca’s and Wernicke’s areas did not alter after aICSL (p = 0.60) and aICSR (p = 0.09). Accordingly, deficits on recall verbal memory after aICS might be accompanied with enhanced activation in secondary language regions. The correlation between significantly altered FCs and recall verbal memory function was shown in Figure 3. In the anterior circulation, FC between right superior medial frontal gyrus and left lingual gyrus in aICSL group showed a high correlation with recall verbal memory (r = -0.559, p=0.010). In the posterior circulation, FC between right middle temporal pole gyrus and right cerebellum8 in aICSR group showed a significant correlation with recall verbal memory (r=-0.395, p=0.028). The underlying functional remodeling in anterior and posterior circulation after aICS is demonstrated in Figure 4. In aICSL group, long cross-hemisphere compensation showed correlation with recall verbal memory. He et al. also reported that patients will be observed with cross-hemisphere connection after aICSL. [14] In aICSR group, inter-hemisphere connection between cerebrum and cerebellum is related to recall verbal memory. Carlson et al. revealed compensation from contralateral posterior circulation in aICSR group.[15]Conclusions
In this study, we evaluated the verbal memory impairment and changed FCs in anterior and posterior circulation territory after aICSL and aICSR. Patients with aICSL or aICSR also showed deficits on recall verbal memory. After aICS, disrupted perfusion altered FCs within secondary language regions instead of primary language regions. Cross-hemisphere connection in anterior circulation and inter-hemisphere connection in posterior circulation were identified in aICSL and aICSR, respectively. Patients with aICSL and aICSR were identified with dissimilar pattern of anterior- and posterior-circulation functional remodeling.Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-010-022-MY3).References
1. Chen, Y.F., et al., Alterations of cerebral perfusion in asymptomatic internal carotid artery steno-occlusive disease. Sci Rep, 2017. 7(1): p. 1841.
2. Cheng, H.L., et al., Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke, 2012. 43(10): p. 2567-73.
3. Chang, C.C., et al., Validating the Chinese version of the Verbal Learning Test for screening Alzheimer's disease. J Int Neuropsychol Soc, 2010. 16(2): p. 244-51.
4. Nagata, T., et al., Non-normalized individual analysis of statistical parametric mapping for clinical fMRI. Neurol India, 2011. 59(3): p. 339-43.
5. Tzourio-Mazoyer, N., et al., Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 2002. 15(1): p. 273-89.
6. Kozasa, E.H., et al., Meditation training increases brain efficiency in an attention task. Neuroimage, 2012. 59(1): p. 745-9.
7. Mechelli, A., et al., Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci, 2000. 267(1455): p. 1909-13.
8. Maguire, E.A., C.J. Mummery, and C. Büchel, Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus, 2000. 10(4): p. 475-82.
9. Chen, S.H. and J.E. Desmond, Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia, 2005. 43(9): p. 1227-37.
10. Wang, T., et al., Impairments in Brain Perfusion, Metabolites, Functional Connectivity, and Cognition in Severe Asymptomatic Carotid Stenosis Patients: An Integrated MRI Study. Neural Plast, 2017. 2017: p. 8738714.
11. Tuo, J., et al., Disrupted Topological Organization of Functional Networks in Asymptomatic Carotid Plaque Without Significant Carotid Stenosis: A Resting-State fMRI Study. Front Hum Neurosci, 2021. 15: p. 685763.
12. Kencht S, D.M., Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H, Language lateralization in healthy right-handers. Brain, 2000. 123 ( Pt 1):74-81.
13. Hartwigsen, G. and D. Saur, Neuroimaging of stroke recovery from aphasia - Insights into plasticity of the human language network. Neuroimage, 2019. 190: p. 14-31.
14. He, S., et al., Altered functional connectivity is related to impaired cognition in left unilateral asymptomatic carotid artery stenosis patients. BMC Neurol, 2021. 21(1): p. 350.
15. Carlson, H.L., et al., Functional connectivity of language networks after perinatal stroke. Neuroimage Clin, 2019. 23: p. 101861.
Figures