2860
Voxel-mirrored homotopic connectivity is impaired in cortical stroke but compensatory in subcortical stroke: a resting-state fMRI study1Yichang Central People's Hospital and The First College of Clinical Medical Science, China Three Gorges University, Yichang, China, 2Institute of Medcical Imaging, China Three Gorges University, Yichang, China, 3Department of Radiology, Zhongnan Hospital of Wuhan University, Wuhan University, Wuhan, China, 4Philips Healthcare, Shanghai, China, 5Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Stroke, fMRI (resting state)
The alteration patterns of bi-hemispheric coordination between homologous areas in the whole brain of acute ischemic stroke patients with different infarct sites remains unclear. In the analysis of the voxel-mirrored homotopic connectivity (VMHC) among patients with frontoparietal lesion (G1), radiation coronal lesion (G2) and basal ganglia lesion (G3), VMHC was significantly decreased in G1 compared with G2 and G3. The impaired regions, such as precentral gyrus and postcentral gyrus, were part of the sensorimotor and default mode network. In contrast, VMHC mostly increased in patients with subcortical stroke, which indicates compensation rather than impairment of bi-hemispheric coordination.Introduction
Homotopic cortical areas in both hemispheres play a crucial role in neuroplasticity and in the reorganization of the brain. 1 It is not well characterized that how the patterns of bi-hemispheric coordination between homologous areas alter in the whole brains of acute ischemic stroke patients with different Infarct sites. Voxel-mirrored homotopic connectivity (VMHC) reflects the synchrony of spontaneous neural activity in bilateral hemispheric architecture between symmetrical regions. It can reliably and reproducibly measure the interhemispheric communication. 2 We hypothesized that VMHC has different damage patterns in acute ischemic stroke patients with different infarct sites. The purpose of this study is to explore the specific VMHC damage patterns in patients with different sites of acute ischemic stroke with motor dysfunction and in healthy controls (HCs).Methods
61 patients with acute ischemic stroke(G1: Frontoparietal lesions, 15 cases; G2: radiation coronal lesions, 16 cases; G3: basal ganglia lesions, 30 cases)and 61 comorbidities- and demographically- matched HCs were enrolled in this study. All patients underwent the neurobehavioral assessments and the resting-state functional magnetic resonance imaging (rs-fMRI) scans. High-resolution structural brain images were acquired by 3.0T Ingenia system (Philips Healthcare, Best, The Netherlands). VMHC was calculated by REST software (http://resting-fmri.sourceforge.net). The individual VMHC maps were converted to z values using a Fisher z-transformation to improve the normality. The individual z-maps were entered into a random effect two-sample t-test with the global VMHC as covariate in a voxel-wise manner to identify the difference in VMHC between groups (familywise error rate corrected, P<0.005 and cluster>20).Results
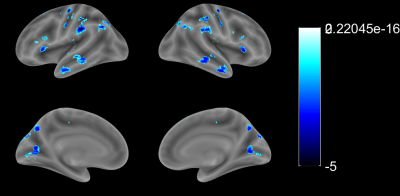
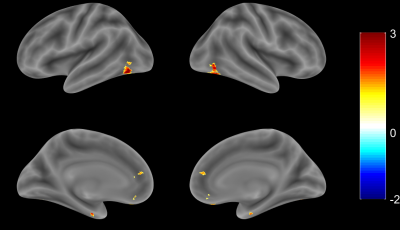
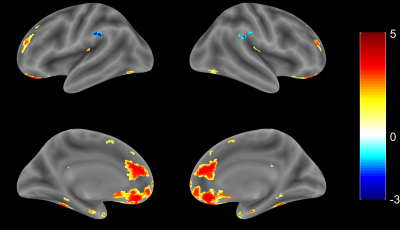
1. Compared with the HCs, G1 displayed lower VMHC in the precentral gyrus, postcentral gyrus, superior frontal gyrus, inferior frontal gyrus, superior temporal gyrus, middle temporal gyrus, supramarginal gyrus, angular gyrus, calcarine fissure and surrounding cortex and superior occipital gyrus (Fig. 1); 2. Compared with the HCs, G2 displayed higher VMHC in the medial of superior frontal gyrus and inferior occipital gyrus (Fig. 2); 3. Compared with the HCs, G3 displayed higher VMHC in the medial orbital of superior frontal gyrus, anterior cingulate gyrus, insula lobe, and fusiform gyrus but lower VMHC in the supramarginal gyrus (Fig. 3).Discussion
Previous studies have shown that there are significant regional differences in correlations between homotopic hemispheres of healthy volunteers. 3 Specifically, there is a gradient of correlation between the cerebral hemispheres, and the correlation of the primary sensory-motor cortex is the highest. In our study, the VMHC in precentral gyrus and postcentral gyrus, which are important components of the sensorimotor network and the default mode network, were reduced in G1 compared with the HCs. This result, which is in line with the findings from previous study, 4 suggests that the functional connections between the components of the sensorimotor network and the default model network in patients with acute cortical infarction are decreased. This impairment might be related to motor, cognitive dysfunction, anxiety, or depression after stroke. 5,6 In contrast, after acute subcortical infarction, VMHC increased in most of the areas analyzed except for bilateral superior marginal gyrus in G3. The increasing pattern was seen in superior frontal gyrus and suboccipital gyrus in G2, and in medial orbital area, anterior cingulate gyrus, insular lobe, and fusiform gyrus in G3. The increasing pattern might link to emotional control, memory, advanced cognitive, and visual function reorganization after acute infarction. 7-9Conclusion
Our results suggest that the alteration pattern of VMHC in acute cortical stroke is different from that of acute subcortical stroke, as the former links to impairment and the latter indicates compensatory effect of brain coordination function. The VMHC was significantly decreased in acute cortical stroke compared with subcortical stroke and the affected brain regions, such as precentral gyrus and postcentral gyrus, were important components of sensorimotor network and default mode network. This study provides additional information for the understanding of the sophisticated functional changes after acute ischemia stroke, which is the basement of efficient patient care.Acknowledgements
We are grateful to our patients and their families for their continued support for our study.References
1. Wu L, Wang C, Liu J, et al. Voxel-Mirrored Homotopic Connectivity Associated With Change of Cognitive Function in Chronic Pontine Stroke. Front Aging Neurosci. 2021;13:621767.
2. Zhu H, Qin R, Cheng Y, et al. The Enhanced Interhemispheric Functional Connectivity in the Striatum Is Related to the Cognitive Impairment in Individuals With White Matter Hyperintensities. Front Neurosci. 2022;16:899473.
3. Stark DE, Margulies DS, Shehzad ZE, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28(51):13754-13764.
4. Shan Y, Wang YS, Zhang M, et al. Homotopic Connectivity in Early Pontine Infarction Predicts Late Motor Recovery. Front Neurol. 2018;9:907.
5. Chen J, Sun D, Shi Y, et al. Altered static and dynamic voxel-mirrored homotopic connectivity in subacute stroke patients: a resting-state fMRI study. Brain Imaging Behav. 2021;15(1):389-400.
6. Yao G, Li J, Liu S, et al. Alterations of Functional Connectivity in Stroke Patients With Basal Ganglia Damage and Cognitive Impairment. Front Neurol. 2020;11:980.
7. Xu P, Chen A, Li Y, Xing X, Lu H. Medial prefrontal cortex in neurological diseases. Physiol Genomics. 2019;51(9):432-442.
8. Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and Function of the Human Insula. J Clin Neurophysiol. 2017;34(4):300-306.
9. Weiner KS, Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016;83:48-62.
Figures