2859
Microstructral and Functional Changes of brain in Pontine Infarction: A Combined Arterial Spin Labeling and Diffusion Tensor Imaging Study
Peipei Chang1, Peng Sun2, and Yanwei Miao1
1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Philips Healthcare, BeiJing, China
1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2Philips Healthcare, BeiJing, China
Synopsis
Keywords: Stroke, Perfusion, Arterial Spin Labeling
Pontine infarction (PI) is very common in posterior circulation infarction, which can lead to behavioral dysfunctions, such as motor and cognition impairment. However, secondary changes in microstructure after PI in the whole brain remain unclear. This study aimed to explore the changes in cerebral blood flow (CBF) and fractional anisotropy (FA) in PI patients and to better understand the whole brain microstructure changes. We found PI could lead to extensive white matter tracts impairment in most of the whole brain regions and perfusion changes in some brain regions.Introduction
Pontine infarction (PI) is very common in posterior circulation infarction, which can lead to behavioral dysfunctions, such as motor and cognition impairment1. However, little is known about the microstructure and cerebral blood flow changes in different brain regions in patients with PI2. Most previous infarction studies focused on patients who experienced infarction of the middle cerebral artery3. Diffusion tensor imaging (DTI) has been used extensively to evaluate the microstructure of white matter (WM) in vivo. Arterial spin labeling (ASL) is a non-invasive MRI technology that can quantify cerebral blood flow (CBF) without a contrast agent and is sensitive to perfusion changes. Based on the abovementioned knowledge, in this study, we aim to investigate the CBF and fractional anisotropy (FA) values difference between the PI and the healthy control (HC) group in different brain regions and provide more information on brain structural and functional alternation induced by PI.Methods
A total of 15 subjects with a single infarction mainly located in the PI and 18 healthy control (HC) subjects were recruited. MRI data for all subjects were acquired using a 3.0T MRI scanner (Ingenia CX, Philips Healthcare, Best, the Netherlands). Multimodal MRI protocol included conventional scan and DTI (TR/TE=6000/92; FOV=256×256 mm2; voxel size=2.00×2.00×2.00 mm3; scan time of 403s), ASL (TR/TE=4252/19; FOV=220×220 mm2; voxel size=3.44×3.50×3.50 mm3; scan time of 434s).Track‐based spatial statistics (TBSS) is a DTI analysis provided by FSL [FMRIB Software Library], which performs a whole‐brain voxelwise analysis of FA values. TBSS was performed according to standard processing pipelines. The covariates included sex and age. The significance level of FA is p<0.05 (5000 permutations, strong control of Family-wise error (FEW) correction for multiple comparisons correction using the threshold-free cluster enhancement). Finally, averaging FA values of the whole brain between the groups were obtained and displayed using the JHU white-matter tractography atlas. CBF images were processed by MATLAB R2013b (MathWorks, Natick, Mass) and SPM8 (Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm). Two-sample t-tests based on whole brain mask were performed with age, and gender as covariates using SPM8. The significance threshold was set to FWE correction at the cluster level (p < 0.05). The Anatomical Automatic Labeling (AAL) template was used to extract the CBF values in different brain regions.Results
1. FA Changes Between PI and HCComparisons between the two groups showed that FA in PI was lower than HC in a wide range of WM tracts, including anterior thalamic radiation, corticospinal tract, cingulum, forceps major, forceps minor, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, uncinate fasciculus (Figure 1).
2. CBF Changes Between PI and HC
The CBF values in the bilateral Putamen, Postcentral, Thalamus, Insula, Rolandic_Oper, Pallidum, Caudate, Precentral, SupraMarginal, Temporal_Sup, Cingulum, Supp_Motor_Area, Precuneus were increased markedly in the PI compared with HC (Table 1, Figure 2), and decreased in Supp_Motor_Area, Frontal_Sup, Frontal_Mid, Precentral, Frontal_Sup_Medial, Paracentral_Lobule, Postcentral, Cerebellum, Temporal_Inf, Fusiform (Figure 3).
Discussion
In this study, we observed that the FA values of most white matter regions in the whole brain were lower than those of the normal control group. This is consistent with the previous studies based on chronic cerebral infarction4, indicating that not only the adjacent tissue but also the remote regions will suffer from white matter structure damage after cerebral infarction. Our research results show that the CBF values of the frontal lobe and cerebellum mainly decreased after infarction, representing the ischemic state of brain tissue. We also found that patients with PI showed higher CBF values in the contralateral frontal lobe, temporal lobe, and basal ganglia. Some researchers have investigated CBF patterns of chronic PI by using ASL and have found that patients with PI also showed higher CBF values in the contralateral inferior frontal gyrus and lower CBF values in the bilateral cerebellum; moreover, the CBF values in the contralateral cerebellum were closely associated with motor function scores5. At present, the detailed mechanisms underlying CBF changes in local brain regions remain unclear, some studies demonstrated that neural activity is strongly associated with CBF, and the balance between neural activity and CBF is disrupted after stroke, resulting in hemodynamic abnormalities6. PI will be accompanied by a series of compensatory mechanisms and changes in the whole brain microstructure, which need to be further explored.Conclusion
Extensive white matter tracts impairment and perfusion changes in different brain regions induced by PI were revealed by DTI and ASL.Acknowledgements
No acknowledgement found.References
1.Sang Hee H , Jae-Chan R , Jae-Han B , et al. Isolated pontine infarction versus pontine plus infarction: prevalence, pathogenic mechanism, and outcomes[J]. Journal of neurology, 2022(8):269.2.Jin Y, Bai X, Jiang B, Guo Z, Mu Q. Repetitive Transcranial Magnetic Stimulation Induces Quantified Functional and Structural Changes in Subcortical Stroke: A Combined Arterial Spin Labeling Perfusion and Diffusion Tensor Imaging Study. Front Hum Neurosci. 2022 Apr 6;16:829688. doi: 10.3389/fnhum.2022.829688. PMID: 35463928; PMCID: PMC9019060.
3.Sagnier S, Catheline G, Dilharreguy B, Linck PA, Coupé P, Munsch F, Bigourdan A, Debruxelles S, Poli M, Olindo S, Renou P, Rouanet F, Dousset V, Berthoz S, Tourdias T, Sibon I. Normal-Appearing White Matter Integrity Is a Predictor of Outcome After Ischemic Stroke. Stroke. 2020 Feb;51(2):449-456. doi: 10.1161/STROKEAHA.119.026886. Epub 2020 Jan 7. PMID: 31906830.
4. Wei Y, Wang C, Liu J, Miao P, Wei S, Wang Y, Wu L, Xu B, Han S, Wei Y, Wang K, Cheng J. Widespread White Matter Microstructure Alterations Based on Diffusion Tensor Imaging and Diffusion Kurtosis Imaging in Patients With Pontine Infarction. Front Aging Neurosci. 2021 Dec 15;13:758236. doi: 10.3389/fnagi.2021.758236. PMID: 34975452; PMCID: PMC8714656.
5. Wang C., Miao P., Liu J., Wei S., Guo Y., Li Z., et al. (2019). Cerebral blood flow features in chronic subcortical stroke: lesion location-dependent study. Brain Res. 1706 177–183. 10.1016/j.brainres.2018.11.009
6. Bohm M., Chung D. Y., Gomez C. A., Qin T., Takizawa T., Sadeghian H. (2020). Neurovascular coupling during optogenetic functional activation: local and remote stimulus-response characteristics, and uncoupling by spreading depression. J. Cereb. Blood Flow Metab. 40 808–822. 10.1177/0271678x19845934
Figures
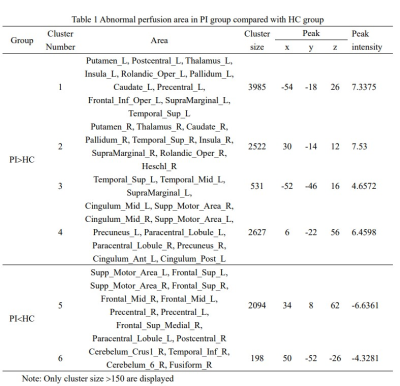
Table 1 Abnormal perfusion area in PI group
compared with HC group
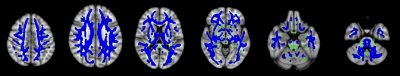
Figure 1 TBSS analyses showed abnormal white
matter tracts in PI. Blue indicated white matter tracts with significantly
decreased parameter values. Green represented the mean FA skeleton of all
subjects.
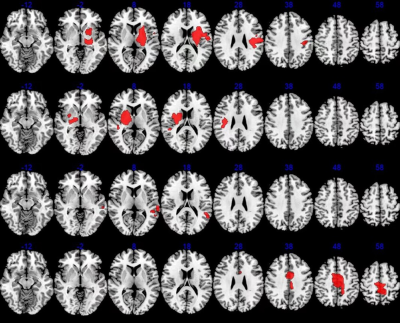
Figure
2 Abnormal perfusion in PI. Red indicated areas with significantly increased CBF
values.
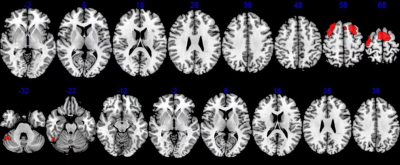
Figure
3 Abnormal perfusion in PI. Red indicated areas with significantly decreased
CBF values.
DOI: https://doi.org/10.58530/2023/2859