2858
Evaluation of altered brain activity in type 2 diabetes by multiple indices of brain function: a resting-state functional MRI study
Ge Zhang1, Xianchang Zhang2, and Meiyun Wang1
1Radiology, Henan Provincial People's Hospital, Zhengzhou, China, 2MR Collaboration, Siemens Healthineers Ltd, Beijing, China
1Radiology, Henan Provincial People's Hospital, Zhengzhou, China, 2MR Collaboration, Siemens Healthineers Ltd, Beijing, China
Synopsis
Keywords: Brain Connectivity, fMRI (resting state)
We combined multiple rs-fMRI indices to investigate the abnormal functional changes among T2DM patients. 52 T2DM patients and matched control participants were enrolled in this study. The functional abnormalities primarily involved motor and sensory function areas. More brain regions were revealed by the combination strategy than any single method and the indices of rs-fMRI showed significantly concordant alterations in most abnormal brain regions. Correlations of between clinical parameters and rs-fMRI indices were found. The current study demonstrated an enriched picture showing the brain functional alterations reflected by the combination of multiple indices in T2DM patients.Introduction
Type 2 diabetes mellitus (T2DM) has been consistently associated with an increased risk of dementia and Alzheimer's disease over the entire lifespan, primarily manifesting as mild cognitive impairment, memory decline, reduced information processing speed and other symptoms which can be used as progressive clinical hallmark of dementia1,2. At present, although a large body of studies has been carried out to explore the relationship between brain functional changes and cognitive impairment in T2DM patients, the pathophysiological mechanism of T2DM-related brain damage stays a puzzle to solve3. In this study, we combined multiple rs-fMRI indices to investigate the abnormal functional changes among T2DM patients and to gain fresh insight of underlying mechanisms of cognition impairment induced by T2DM.Methods
52 T2DM patients and age-, sex- matched control participants were included in this study. All participants received MRI examination using a Siemens 3.0T Prisma scanner with a Standard Siemens 8-channel head coil. Structural images were acquired using the three dimensional T1-weighted spoiled gradient recalled echo sequence. Functional images were acquired using gradient-echo planar imaging sequence. Anthropometric and medical information were recorded from all participants, including age, gender, weight, height, duration of diabetic disease, glycosylated hemoglobin (HbAlc) , fasting plasma glucose (FPG), total cholesterol, triglyceride (TAG), high density lipoprotein (HDL), low density lipoprotein (LDL), systolic blood pressure (SBP) and diastolic blood pressure (DBP). The cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) for each subject. Resting-state fMRI data preprocessing was carried out using the toolbox Data Processing & Analysis for Brain Imaging (DPABI). Amplitude of low frequecny fluctuations (ALFF), regional homogeneity (ReHo) and voxel-mirrored homotopic connectivity (VMHC) values were calculated to represent the status of spontaneous neural activity. The independent-sample t test was used to conduct the comparison of the indices between the two groups. Correlations between rs-fMRI indices and clinical parameters were calculated.Results
Compared with control subjects, the rs-fMRI indices of T2DM patients showed significantly concordant alterations in several brain areas including bilateral cerebellum posterior lobe, left inferior temporal gyrus, parahippocampal gyrus and supplementary motor area. Although no abnormal brain region was detected by all three indices at the same time, the indices presented significant correlation in most regions revealed by each single method. In the T2DM group, the fasting glucose was correlated with decreased neural activity in the surrounding areas of left insula and inferior frontal gyrus. While the MoCA results did not differ between two groups, the cognition performance was negatively correlated with mean values of ReHo extracted from left occipital lobe and superior cerebellum cortex, and the mean values of VMHC extracted from left caudate and precentral gyrus.Discussion
While conducting rs-fMRI analysis, it may be confusing to decide which measurement is more preferably to reveal the intrinsically abnormal brain regions with damaged function. It’s an inevitable barrier to translate kinds of analyzing results of T2DM-induced cognitive impairment into clinical practise. In this study, we tried to explore the abnormalities of functional spontaneous activity over the whole brain in T2DM patients versus control participants by combining rs-fMRI indices including ALFF, ReHo and VMHC. The functional abnormalities primarily involved motor and sensory function areas, which were located in cerebellum, SMA, ITG and occipital lobe. In terms of the potential correlation between rs-fMRI indices, more brain regions with disordered neural activity were determined via the integrated analyses than any single method. And the ITG.L, bilateral inferior cerebellum cortex, and SMA.L were overlapped in the single results, indicating a more robust and important role in the functional changes of the brain. Meanwhile, correlations between clinical variables and rs-fMRI indices were presented in brain regions involving sensory-motor network and low-level perception, further supporting our findings.Conclusion
The analysis of combining the rs-fMRI indicators ALFF, ReHo and VMHC revealed more abnormal brain regions than any single method of the three ones, which was conductive to comprehensive detection and focusing on the probably key brain areas. Meanwhile, the indicators were correlated in the located brain areas, further proving the necessity of the combination of multiple indicators. Our combined analysis demonstrated widespread alterations in the brain regions mainly related to motor and sensory processing, which were indirectly involved in cognitive function. These findings were further verified by association analysis between rs-fMRI indicators and clinical variables in the insula, inferior temporal gyrus, occipital lobe and cerebellum. Comparing with previous studies, these results reflect the heterogeneity of brain function changes in T2DM patients, which may be different stages of diabetes-related cognitive impairment. In future studies, introducing micro-scale imaging information (e.g. the positron emission tomography) and conducting longitudinal studies may provide a coherent process of dynamic development of T2DM related cognitive impairment.Acknowledgements
NoneReferences
1. Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758-766. 2. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653-666. 3. Yao L, Yang C, Zhang W, et al. A multimodal meta-analysis of regional structural and functional brain alterations in type 2 diabetes. Front Neuroendocrinol. 2021;62:100915.Figures
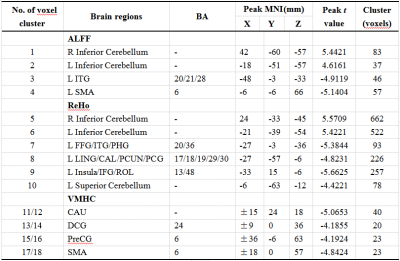
Figure 1. Differences in ALFF, ReHo and VMHC values between the patient and control groups.Comparisons were performed at p < 0.05, corrected for multiple comparisons with GRF method. IFG, Inferior frontal gyrus; ITG, Inferior temporal gyrus; SMA, Supplement motor area; LING, lingual gyrus; CAL, Calcarine fissure and surrounding cortex; DCG, Median cingulate and paracingulate gyri; PCUN, Precuneus; PCG, Posterior cingulate gyrus; FFG, Fusiform gyrus; ROL, Rolandic operculum; CAU, Caudate nucleus; PHG, Parahippocampal gyrus; PreCG, Precental gyrus.
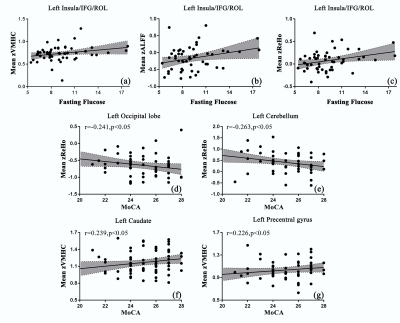
Figure 2. Significant correlations between the mean values of rs-fMRI indices extracted from reported brain regions and clinical parameters in T2DM patients. (a)-(c) showed the mean z values of ALFF, ReHo and VMHC in surrounding areas of insula were correlated with fasting glucose respectively. (d)(e) showed that mean z values of ReHo in left occipital lobe and superior cortex of left cerebellum were negatively correlated with the MoCA scores. (f)(g) showed mean z values of VMHC in left caudate and precenteral gyrus were correlated with the MoCA scores.
DOI: https://doi.org/10.58530/2023/2858