2847
The abnormal functional connectivity of the locus coeruleus can classify migraine without aura patients1The Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University, Wenzhou, China, 2The Hebei General Hospital, Shijiazhuang, China, 3Xingtai people's Hospital, Xingtai, China, 4Philips Healthcare, Shanghai, China, Shanghai, China, 5The Second Affiliated hospital, Hebei Medical University, Shijiazhuang, China
Synopsis
Keywords: Brain Connectivity, Nervous system, migraine
Blood flow to the brainstem region is increased as a hypothesis for migraine. Locus coeruleus (LC) is the main noradrenergic nucleus in the brainstem. However, the functional characteristics of LC in migraine without aura (MwoA) patients are not currently known. Our results demonstrated that patients with MwoA exhibited significant LC FC differences in the brain areas associated with visual and cognitive function. Understanding the changes in the LC brain network in MwoA patients can provide us with new ideas to understand the pathological mechanisms of MwoA.Introduction
The neurovascular hypothesis of migraine implicates a central role of the trigeminovascular system1. Locus coeruleus (LC) is located in the medial nucleus of the trigeminal nerve bundle and the main nucleus in the brainstem that releases norepinephrine. Abnormalities in LC functional are thought to be closely related to migraine2. However, to the best of our knowledge, there are no studies using rsfMRI and FC to explore LC functional connectivity patterns in MwoA patients.Methods
Between March and October of 2014, 28 MwoA patients and 17 HCs were recruited for this study. The International Classification of Headache Disorders, Third Edition (beta version) diagnostic criteria are met by all MwoA subjects (ICHD-3beta). All subjects underwent a functional fMRI scan with an 8-channel head coil on a 3 T MRI scanner (Achieva X-series, Philips Medical, Best, the Netherlands). T2*-weighted echo-planar images were acquired for the functional scan with the following parameters: 35 axial slices, thickness/gap = 4/0 mm, in-plane resolution = 80×80, repetition time (TR) = 2000 ms, echo time (TE) = 35 ms, flip angle = 90°, and FOV = 240×240 mm2. Each functional imaging had 240 volumes. During image acquisition, we instructed patients to remain still and close their eyes. Using an atlas-based method, two separate LC seeds (one per hemisphere) were created on the AAL3 template for each participant (Fig. 1). SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and DPABI v6.0 (http://rfmri.org/DPABI) were used for data preprocessing. General linear model were used to compare whether there were differences in LC brain networks between the two groups. We also utilized logistic regression to explore the role of LC functional networks in the clinical diagnosis of MwoA.Results
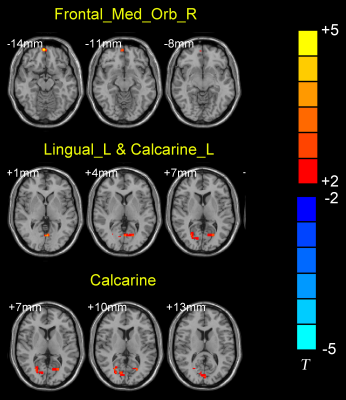
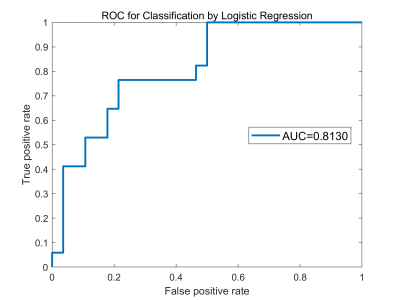
After general linear analysis, MwoA patients displayed increased FC from right LC to the left lingual and calcarine sulcus, as well as to the right frontal medial gyrus/orbit part, when compared with HCs (Fig. 2). The results of the logistic regression showed that the LC FC signals were 81% accurate in distinguishing MwoA from the HCs (Fig. 3).Discussion
The brain regions where we found significant differences were located in the occipital lobe. Experiments in rats showed that significant enhancement of Fos immunoreactivity in the caudal nucleus of the trigeminal nerve, LC, parabrachial nucleus and median suture nucleus after injurious trigeminal nerve stimulation with capsaicin3. Locus coeruleus may also directly modulate trigeminal spinal tract nucleus neurons, leading to alpha2-adrenoceptor-dependent cerebral hypoperfusion, a known trigger for cortical spreading depression (CSD)4. In addition, loss of cortical norepinephrine upstream signal will hyperexcite LC and lower the CSD threshold. While, the CSD hypothesis refers to an inhibitory band of neuroelectrical activity originating in the occipital lobe caused by noxious stimuli, which extends at a rate of 2 to 5 mm/min into the adjacent cortex and is accompanied by spreading oligemia5.Conclcusion
Our results demonstrated that patients with MwoA exhibited significant LC FC differences in the brain areas associated with visual and cognitive function. Understanding the changes in the LC brain network in MwoA patients can provide us with new ideas to understand the pathological mechanisms of MwoA.Acknowledgements
noneReferences
1. Lambert GA, Zagami AS. The mode of action of migraine triggers: a hypothesis. Headache. 2009;49(2):253-75.
2. Poe GR, Foote S, Eschenko O. et al. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci. 2020;21: 644–659.
3. Ter Horst GJ, Meijler WJ, et al. Trigeminal nociception-induced cerebral Fos expression in the conscious rat. Cephalalgia. 2001;21(10):963-75.
4. Vila-Pueyo M, Strother LC, Kefel M, et al. Divergent influences of the locus coeruleus on migraine pathophysiology. Pain. 2019;160(2):385-394. 6. Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9(11):637-44.
5. Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9(11):637-44.
Figures