2846
Disruption of default mode network and salience network dynamics in acute traumatic pain states1Pennsylvania State University College of Medicine, Hershey, PA, United States
Synopsis
Keywords: Brain Connectivity, Neuro, Pain
Persistent and chronic pain following acute musculoskeletal injury is a significant contributor to diminished quality of life. We correlated self-reported pain using the McGill Pain Questionnaire with resting-state brain network connectivity in patients with blunt chest trauma. Results suggest that both inferior parietal and insular cortex connectivity were positively correlated to greater self-reported pain and a region within the salient network showed a negative correlation. These results support the hypothesis that aberrant functioning of brain circuits that assign salience values to stimuli may contribute to pain perception. Understanding abnormal activity/connectivity may identify targets to prevent persistent and chronic pain.
Introduction
Persistent and chronic pain following an acute musculoskeletal injury is a significant contributor to diminished quality of life. To identify predictors of the transition from acute to chronic pain, we have been focusing on psychological, biological, and resting state brain network measures in patients with blunt chest trauma with multiple (>2) closed rib fractures. Using this acute pain model, we investigated two resting state networks implicated in pain processing: the default mode network (DMN)1 and the salience network (SN)2. We hypothesized that connectivity patterns within these networks will be correlated with total scores on the McGill Pain Questionnaire (MPQ).Materials and Methods
Fifteen subjects (13 males, mean age 57.5 yrs.) with blunt chest trauma took part in our ongoing study. We acquired rs-fMRI data on a Siemens 3T MRI system (Magnetom Trio, Siemens Medical, USA) with a 64-channel head coil. A T2*-weighted EPI sequence was used with the following image acquisition parameters: TR / TE / FA = 1200 ms / 30 ms / 50°, FOV = 240 mm x 240 mm, image resolution = 3 mm × 3 mm, 54 slices with a thickness of 3 mm and multi-band acceleration factor of 8. During rs-fMRI scanning, subjects were instructed to lie still with their eyes opened. SPM12 was used to preprocess rs-fMRI data. Independent component analysis (ICA) was performed using software packages developed in-house.Results and Discussion
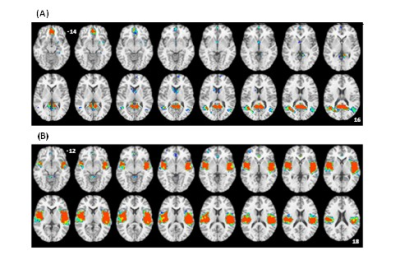
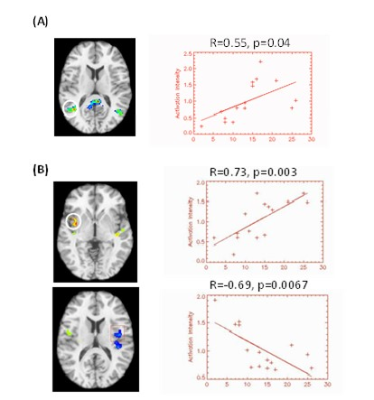
Figure 1 shows the group ICA maps for the salience network and the default mode network. We were able to identify each network clearly at the single subject level. We used these images to create DMN and SN masks that were used in a subsequent correlation analysis (controlling for age) between network connectivity and the total MPQ score. Figure 2 A shows that inferior parietal cortex connectivity positively correlates with total MPQ scores. Similarly, Figure 2 B shows that insular cortex connectivity positively correlates with total MPQ scores. However, we also found a region within the SN mask that showed a negative correlation with total MPQ scores.It has been hypothesized that the DMN is acting as a sentinel by monitoring the external environment3. Therefore, connectivity changes within the DMN may underpin pain monitoring and potentially reduce the ability of other stimuli to attract an individual's attention away from pain. Regarding the SN, our preliminary results are in line with the hypothesis that aberrant functioning of brain circuits that assign salience values to stimuli may contribute to pain perception.
Conclusion
Collectively, our results suggest that a refined understanding of abnormal activity/connectivity of brain regions within the DMN and SN is necessary to effectively target interventions for patients with acute and chronic pain.Acknowledgements
We thank staff members of the Center for NMR Research at Penn State University. This research is supported by the National Institute of Health (R35 GM146774 and 1R01AG070088-01A1) and DOD (CPMRP W81XWH-21-1-0870) grants.References
1. Z. Alshelha Z et al., (2013) The Value of Salience Circuits. Prog Neurobiol 104: 93–105
2. David Borsook et al., (2013) The Value of Salience Circuits. Prog Neurobiol 104: 93–105
3. Buckner RL. Et al., (2008) The brain's default network anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38.