2844
Therapy effects on the structural connectome of children treated for medulloblastoma1Diagnostic Imaging, St. Jude Children's Research Hospital, Memphis, TN, United States, 2Biostatistics, St. Jude Children's Research Hospital, Memphis, TN, United States, 3Oncology, St. Jude Children's Research Hospital, Memphis, TN, United States, 4Radiation Oncology, St. Jude Children's Research Hospital, Memphis, TN, United States
Synopsis
Keywords: Brain Connectivity, Cancer
This study reports on the effects of pediatric medulloblastoma therapy in a population of 68 subjects. Structural connectivity was evaluated after surgery and again at the end of both radiation and chemotherapy. Linear models were written for the difference between these two time points controlling for age and risk arm. Fourteen significant edges were found connecting the basal ganglia to the dorsolateral prefrontal and prefrontal cortices as well as the cingulate. These regions are part of the executive function network and may point to the mechanisms of decreases in neurocognitive performance.PURPOSE
Five-year survival for medulloblastoma (MB), the most common pediatric brain tumor, remains approximately 80% for standard risk and 60% for high-risk subjects.1 White matter (WM) microstructural damage has been observed in patients treated for MB using combined modality therapy including surgery, post-operative craniospinal irradiation (CSI), and post-irradiation systemic chemotherapy. Abnormalities have been measured after surgery and prior to adjuvant therapy and decreasing normal appearing WM volumes have been measured after the completion of all therapy.2 Because there is an established link between therapy intensity and neurocognitive decline3, we sought to assess structural connectivity before and after adjuvant therapy hypothesizing that smaller connections would be measured at the completion of therapy.PATIENTS AND METHODS
Subjects included in the current report were those enrolled on a multi-institution trial (NCT01878617) at a single institution. Treatment included maximal surgical resection, risk-adapted craniospinal irradiation (CSI), and risk-stratified adjuvant chemotherapy based on molecular and clinical factors. Two time points were used in this study: baseline after surgery and 12 months after diagnosis. The 12-month time point corresponded to the completion of radiation therapy and chemotherapy. Eligible subjects had both MRI examinations with the same diffusion tensor imaging (DTI) acquisition parameters and completed the planned treatment course. There were 68 patients with a median age of 10.5 years at baseline, range, 5.3-22.8 years. There were 28 females and 40 males. The cohort was further characterized as wingless (WNT, n=12) molecular phenotype, Sonic hedgehog (SHH, n=14), and Non-WNT Non-SHH (n=42). Ten subjects were low risk (15 Gy CSI), 29 were standard risk (23.4 Gy CSI), and 29 were high risk (36 Gy CSI). Imaging and treatment protocols were approved by the local Institutional Review Board, and written informed consent was obtained.Anatomic imaging was collected on 3.0T whole-body systems (Siemens Medical Systems, Iselin, NJ) for all subjects using a 3D T1 weighted MPRAGE sequence [TR/TE/TI = 1980/2.26/1100 ms] with 1mm isotropic resolution. These isotropic images were processed using the FreeSurfer pipeline (http://surfer.nmr.mgh.harvard.edu/)4, with manual inspection and editing completed to ensure quality anatomic parcellation were achieved. A combined volumetric and surface-based registration was then used to bring multimodal parcellations (HCP_MMP1.0) back into the patient space as seeds and targets for tracking.5 DTI was collected with either a 30 direction, two average acquisition [TR/TE=14000/120ms, b=700s/mm2, FOV=230, 1.8x1.8x2mm resolution], or a 64 direction, two reverse blip acquisition [TR/TE=4000/77.4ms, b=1500s/mm2, FOV=230, MB=4, 1.8x1.8x1.8mm resolution] based on the time of the examination. Diffusion data were processed with the MRtrix3 Software (http://www.mrtrix.org/).6
The two-dimensional connectivity matrices were converted into 1D vectors that included the upper half of the symmetrical triangular matrix. The difference for each edge was calculated by subtracting the baseline examination from the 12-month examination. To control for the effects of age and risk stratification on the connectivity difference, a linear model was written as:
Delta 12month - Baseline = β0 + β1 Age + β2 Risk + ε
where Delta is the connectivity difference for a given edge, Age is the age in years of the subject at the baseline examination, and Risk was one of the three different risk stratifications. A false discovery rate (FDR) adjusted7 p value of 0.05 was considered significant.
RESULTS
A total of 71,631 linear models were fitted to describe the connectivity change for the edges between the 12-month and baseline examinations. A significant edge represents a connection that is changed between the two examinations after considering both age and risk stratification in the model. After controlling for the false discovery rate, only 14 edges were statistically significant (Table 1). All 14 of these edges demonstrated a negative difference, representing lower connectivity at the 12-month examination compared to the baseline examination. Twelve of the 14 edges had a node in the basal ganglia with connections to the dorsolateral prefrontal or prefrontal cortex as well as the cingulate (Figure 1).DISCUSSION
This study continues to expand our knowledge of central nervous system effects of current pediatric medulloblastoma therapy. Previous work has shown a level of damage due to surgery alone2, and additional damage is expected immediately after radiation therapy. The results presented in this study suggest that there is damage to structural connections even after correcting for the effects of age and treatment risk, a surrogate of CSI radiation dose. These changes were observed when comparing the baseline examination occurring after surgery but before radiation or chemotherapy to the first examinations after the end of both potentially toxic therapies. The edges identified connect primarily basal ganglia regions to the frontal lobe and cingulate, regions known to be critical in executive function tasks. It will be important to associate the neurocognitive performance in these subjects to the measured changes in these connectivity metrics.CONCLUSION
Structural connectivity changes seen after radiation and chemotherapy can help to elucidate the foundations between these therapy effects and the functional regions affected. Identification of these regions could aid in the understanding of how damage can manifest itself on neurocognitive performance.Acknowledgements
We acknowledge the valuable contributions of Rhonda Simmons and Kathy Jordan, advanced signal processing technicians, and funding in part by the Cancer Center Support Grant P30 CA-21765 from the National Cancer Institute, and ALSAC.
References
1. Gajjar A, Robinson GW, Smith KS, et al. Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03). J Clin Oncol. 2021; 39(7):822-835.
2. Glass JO, Ogg RJ, Hyun JW, et al. Disrupted development and integrity of frontal white matter in patients treated for pediatric medulloblastoma. Neuro Oncol. 2017; 19(10):1408-1418.
3. Acharya S, Guo Y, Patni T, et al. Association Between Brain Substructure Dose and Cognitive Outcomes in Children With Medulloblastoma Treated on SJMB03: A Step Toward Substructure-Informed Planning. J Clin Oncol. 2021:JCO2101480.
4. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000; 97(20):11050-11055.
5. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016; 536(7615):171-178.
6. Song R, Glass JO, Reddick WE. Modified Diffusion Tensor Image Processing Pipeline for Archived Studies of Patients With Leukoencephalopathy. J Magn Reson Imaging. 2021; 54(3):997-1008.
7. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995; 57(1):289-300.
Figures
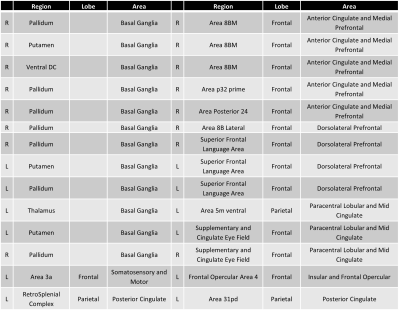
Table 1 The nodes that delimit the statistically significant edges that changed between the baseline and 12-month examinations after controlling for age and risk arm.
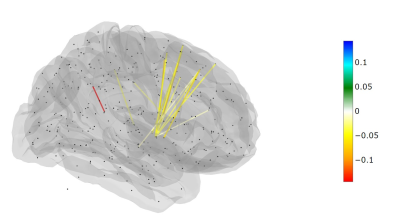
Figure 1. A glass brain viewing the right side of the brain and depicting the statistically significant edges representing change between the baseline and 12-month examinations after controlling for age and risk arm. The color of the edge is scaled to represent the average size of the significant difference between the examinations.