2839
Time- and Signal-Efficient 3T Ultra-High-Resolution Imaging of the Ex Vivo Cerebellum and Entire Human Brain1Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, Faculty of Medicine, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurological Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland, 3Division of Radiological Physics, Dept. of Radiology, University Hospital Basel, Basel, Switzerland, 4MR-Research in Neurosciences, Department of Cognitive Neurology, University Medical Center Göttingen, Göttingen, Germany, 5Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany
Synopsis
Keywords: Multiple Sclerosis, Ex-Vivo Applications
Ex Vivo MRI of the entire human brain facilitates new and fascinating insights into cerebral and cerebellar morphology and pathology. One key factor for achieving ultra-high spatial resolutions is a signal- and time-efficient MRI sequence, particularly, at 3T field strength. Though counterintuitive at first notion, we suggest a “slow” balanced steady-state free precession (bSSFP) approach with phase-cycling and very low receiver bandwidth (“LoBa-bSSFP”) as a highly signal- and time-efficient scheme for ex vivo acquisitions. LoBa-bSSFP can support spatial resolutions as high as 98-microns isotropic for covering the entire cerebellum on a common 3T MR system.INTRODUCTION
Ultra-high resolution imaging (URI) of the entire human ex vivo brain has raised a lot of interest previously. Extended acquisition times under lab-controlled ex vivo conditions allow resolving neuro morphology and pathology such as multiple sclerosis (MS) in an MRI domain not possible before 1-5.Although early work already used the most signal-efficient balanced steady-state free precession (bSSFP) approaches 1,2, recent work achieved isotropic resolutions up to 100µm (7T) and 160µm (3T) with T2* weighted RF spoiled gradient echo (FLASH) sequences with very low receiver bandwidth 4,5. T1 weighted FLASH approaches were also presented 6,7. FLASH sequences combine the advantages of good SNR, superb contrast, low MR system demand, and very good long-term stability 5.
Last year, it was demonstrated that 3T bSSFP based acquisitions are feasible up to 200µm isotropic 8; nevertheless, all work to date followed the “common bSSFP rationale” of using high receiver bandwidths, maximal gradient amplitudes, and minimally short repetition times TR.
The purpose of this work is to demonstrate that – under ex vivo conditions – it is rather advantageous to realize a “slow” bSSFP approach with longer TR, weaker gradients, and a very low receiver bandwidth. This “LoBa-bSSFP” approach currently facilitates resolutions as high as 98µm isotropic for cerebellar imaging - with a standard 3T setup.
METHODS
Specimen preparation and experimental setupTwo whole brains of patients diagnosed with MS have been fixated directly in 10% formalin within 24h after death (MS1: f, 70y, DD 36y; MS2: m, 63y, DD unknown). For MRI acquisition, the brains were positioned in a dome-shaped container 9-11 and immersed in a fluorinated oil. Air bubbles were removed using a vacuum pump. A 3T whole-body MR system with a standard 20-channel head coil was employed.
Acquisition
For bSSFP based URI, an in-house developed bSSFP sequence with RF phase-cycling 12 functionality was employed, which circumvents typical restrictions like maximal 3D matrix size and minimal receiver bandwidth, therefore, exploiting the full vendor’s on-the-fly reconstruction capability. Based on pre-experiments, the optimal bSSFP flip angle 13 was determined to be ~36deg.
Whole-brain protocol 200μm isotropic: matrix 784x960x640, TR/TE = 17.0ms/8.5ms, bandwidth=120Hz/Px, flip angle 60deg for increased T2-weighting, twelve phase-cyclings, TAtotal= 28.5h. Cerebellum protocol 115μm isotropic: matrix 832x936x576, TR/TE = 34.0ms/17.0ms, bandwidth=50Hz/Px, flip angle 42deg, two times six phase-cyclings, TAtotal = 80h. Cerebellum protocol 98μm isotropic: matrix 976x1080x576, TR/TE = 36.0ms/18.0ms, bandwidth=50Hz/Px, flip angle 36deg, acquisitions had four phase-cyclings each time, once full k-space (TAtotal = 32.5h) and twice Half-Fourier encoding (TAtotal =34.5h). Both cerebellum protocols take advantage of slab selection and no-wrap-around in readout direction, thus, realizing a “reduced field-of-view” without aliasing. All protocols acquired transverse 3D slices with A-P readout.
Magnitude and phase images were reconstructed with the standard MR system for each phase-cycled or repeated acquisition, and then complex-averaged offline. An optional, intermediate rigid-body co-registration step utilized Elastix 14.
RESULTS
Figure 1 shows a representative 200µm LoBa-bSSFP acquisition of the entire brain, contrasting the twelve phase-cycled acquisitions with the final image. In this preliminary experiment, a low bandwidth of 120Hz/Px was employed. Hard- and software wise a 160µm isotropic resolution for the entire brain can be supported. Identical window-leveling was used for all MR images; also in Fig. 2, where the first viable boundaries for the suggested LoBa-bSSFP approach were tested: An isotropic 98µm protocol for the cerebellum was tested. The receiver bandwidth is only 50 Hz/Px and the TR as long as 36ms. As shown, image co-registration and complex-valued averaging are essential for ensuring the full potential.Figures 3 and 4 illustrate well that the isotropic 115µm LoBa-bSSFP approach can reveal the complex cerebellar fine structure.
DISCUSSION and CONCLUSION
The presented work initially follows the concept of Weigel et al 5, which is appealing due to its simplicity of utilizing a clinical 3T MR system with a standard head coil and a simple MRI sequence with very low receiver bandwidth to maximize SNR per unit time. Repeated acquisitions, harnessing the full online image reconstruction capabilities of the MR system, are then averaged up.Our suggested rationale for a highly time- and signal-efficient 3T ex vivo MRI approach is similar, though it may appear counterintuitive for a bSSFP approach. However, we believe that this notion is biased by the very different in vivo conditions.
Fig. 1 already demonstrates the performance of a preliminary 200µm LoBa-bSSFP acquisition. Further refinements are necessary, however, for harnessing the full potential of a 98µm cerebellar acquisition (Fig. 2): Compared to mere averaging of magnitude images 5, it gets essential to average the full complex image data, thus, avoiding the development of a signal noise floor and reduced image contrast. Furthermore, image co-registration may become important. Additionally, Half Fourier encoding, which was originally tried to shorten each scan for lower intra-scan drift, is not advisable (Fig. 2).
Figures 3 and 4 complement the work with the results of a 115µm cerebellar acquisition. Overall, all important banding and drift artifacts could be tackled well with the phase-cycling approach (Figs. 1 to 4).
To conclude, we showed that the presented LoBa-bSSFP approach enables true ex vivo URI at 3T in reasonable time. Besides further investigations, future work will exploit LoBa-bSSFP data for deriving quantitative measures.
Acknowledgements
This work was funded by the Swiss National Fund PP00P3_176984 and supported by the German Ministry of Education (BMBF; KKNMS German competence network for multiple sclerosis).References
1. Pfefferbaum A, Sullivan EV, Adalsteinsson E, et al. Postmortem MR imaging of formalin-fixed human brain. Neuroimage 2004;21(4):1585-95.
2. Miller KL, Stagg CJ, Douaud G, et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 2011;57(1):167-181.
3. Tendler BC, Hanayik T, Ansorge O, et al. The Digital Brain Bank, an open access platform for post-mortem imaging datasets. eLife 11:e73153. doi: 10.7554/eLife.73153
4. Edlow B, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data 2019 Oct 30;6(1):244.
5. Weigel M, Dechent P, Galbusera R, et al. Imaging multiple sclerosis pathology at 160μm isotropic resolution by human whole-brain ex vivo magnetic resonance imaging at 3 T. Sci Rep 2021 Jul 29;11(1):15491. doi: 10.1038/s41598-021-94891-1.
6. Weigel M, Galbusera R, Dechent P, et al. T1 Weighted Postmortem MR Imaging of the Cerebellum at 3T: Preliminary Results between Feasibility and Desire. Proceedings of ISMRM 2022: p2022.
7. Pracht E, Cremer M, Loewen D, et al. High Resolution Postmortem Brain Imaging at 7 Tesla using Parallel Transmission and a Simple Set-Up. Proceedings of ISMRM 2022: p1915.
8. Weigel M, Dechent P, Galbusera R, et al. Exploring Ultra-High Resolution Imaging of the Ex Vivo Whole Brain: Initial Results with Balanced Steady State Free Precession Sequences at 3T. Proceedings of ISMRM 2022: p1914.
9. Luciano NJ, Sati P, Nair G, et al. Utilizing 3D Printing Technology to Merge MRI with Histology: A Protocol for Brain Sectioning. J Vis Exp 2016 Dec 6;(118).
10. Absinta M, Nair G, Filippi M, et al. Postmortem Magnetic Resonance Imaging to Guide the Pathologic Cut: Individualized, 3-Dimensionally Printed Cutting Boxes for Fixed Brains. J Neuropathol Exp Neurol. 2014;73(8):780-8.
11. Griffin AD, Turtzo C, Parikh G, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019;142(11):3550-3564.
12. Bangerter NK, Hargreaves BA, Vasanawala SS, et al. Analysis of multiple-acquisition SSFP. Magn Reson Med. 2004 May;51(5):1038-47. doi: 10.1002/mrm.20052.
13. Scheffler K. On the transient phase of balanced SSFP sequences. Magn Reson Med 2003 Apr;49(4):781-3. doi: 10.1002/mrm.10421.
14. Klein S, et al. IEEE Trans Med Imaging 2010;29:196-205.
Figures
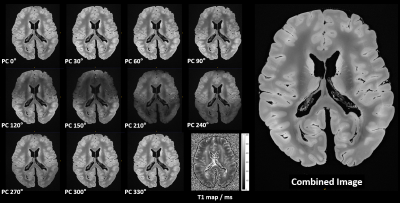
Figure 1
Juxtaposition of 200µm LoBa-bSSFP acquisitions with different phase cyclings (PC) as noted: 11 of 12 are shown, brain MS1. Some acquisitions are affected by broader, hypointense areas, caused by intra-scan B0 drift and susceptibility induced bSSFP banding. The image combination indeed facilitates 3D URI with homogenous signal intensity, high SNR and good soft tissue contrast. The weak circumferential intensity rim with contrast change is a T1 mediated fixation effect (cf. T1 map).
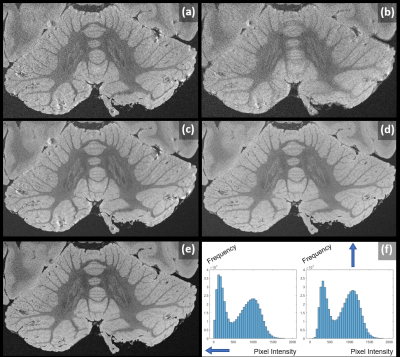
Figure 2
Proof of feasibility for a cerebellum-focused 98µm LoBa-bSSFP acquisition, brain MS2. Later acquisitions (b) are more susceptible to inter-scan drift-induced blurring effects in averaging, compared to earlier ones (a). Proper co-registration leads to strong quality improvement (c). Residual susceptibility artifacts were due to initial Half-Fourier encoding. Full k-space sampling avoids these (d). For such noisy acquisitions, it is important to average full complex image data as done in (e); underlined by the histograms of corresponding pixel intensities (f).
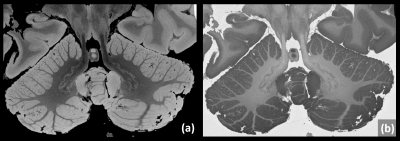
Figure 3
A representative slice of a cerebellum-focused 115µm isotropic LoBa-bSSFP acquistion is shown, which was roughly adapted to the total acquisition time of one long weekend (brain MS2). “Despite” the standard clinical 3T system, the resulting image quality is rather impressing (a). A few locations in the cerebellum show cortical changes: Subpial demyelination is suspected, but needs histopathological confirmation.
Additionally, a simple real part reconstruction depicts a strong soft-tissue contrast (b).
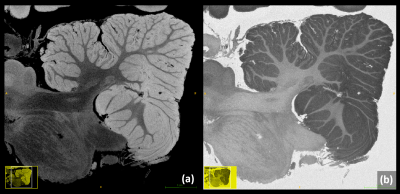
Figure 4
A sagittal reformation of the 115µm LoBa-bSSFP acquisition from brain MS2 is depicted, emphasizing the need for full isotropic URI in 3D. The reformed images also demonstrate a homogenous intensity behavior with a very good soft tissue contrast. In this view, areas of suspected subpial demyelination can be observed even better. Again, a corresponding real part reconstruction provides a strong “inverted contrast” in the cerebellum.