2836
Characterization of Paramagnetic Rim Lesions using quantitative and semi-quantitative MRI modalities1Department of Neurosciences, Biomedicine and Movement sciences, University of Verona, Verona, Italy, 2Neuroradiology Unit, Department of Diagnostics and Pathology, University of Verona, Verona, Italy, 3Department of Information Engineering, University of Padova, Padova, Italy
Synopsis
Keywords: Multiple Sclerosis, Multi-Contrast, paramagnetic rim lesions
Chronic active lesions have been recently investigated in multiple sclerosis (MS) and associated with the detection of paramagnetic rims on susceptibility-based MRI images (filtered phase and quantitative susceptibility mapping). Quantitative MRI approaches have the potential to reveal the degree of tissue damage in paramagnetic rim lesions. In this study a significant increase in T2* relaxation time and Quantitative Susceptibility Mapping (QSM) as well as a decrease in Magnetization Transfer Ratio (MTR) and Magnetization Transfer Saturation (MTSat) was revealed in PRL+ lesions compared to PRL-, thus supporting the use of these MRI metrics to detect tissue damage associated with chronic activity.INTRODUCTION
Multiple sclerosis (MS) is a chronic, demyelinating disease affecting the central nervous system showing focal lesions in both white and grey matter tissue. Neuropathological assessments have identified a subset of lesions, called chronic active lesions, characterized by an accumulation of iron-rich activated microglia at the lesion's border1. The presence of paramagnetic rims detectable on susceptibility-based sequences has become an in-vivo surrogate marker of chronic active lesions2. Although neuropathological assessments evidenced the presence of demyelination and axonal damage in the core of chronic active lesions, a characterization of the MR properties of paramagnetic rim lesions (PRL) cores is still lacking. Quantitative and semi quantitative MRI approaches, being influenced by the underlying tissue composition and microstructure, have the potential to reveal subtle pathological changes for the in-vivo characterization of PRL+. Thus, in this study we aimed to use an MRI protocol comprehending the quantification of T2* relaxation time, quantitative susceptibility mapping (QSM), Magnetization Transfer Ratio (MTR), Magnetization Transfer Saturation (MTSat) and Myelin Water Fraction (MWF) to characterize the core of paramagnetic rim lesions in-vivo.METHODS
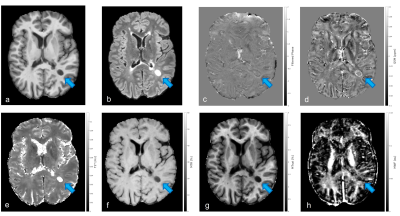
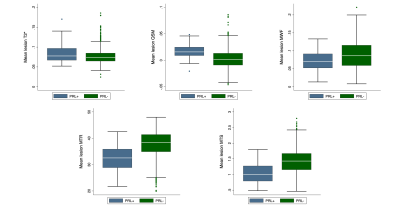
Relapsing MS patients were enrolled at the Multiple Sclerosis Centre of Verona University Hospital and underwent an MRI acquisition carried out on a 3T Ingenia Elition S (Philips Healthcare, Best, The Netherlands) using a 32 channels head coil. The MRI protocol comprehended: 3D T1 MPRAGE (TR/TE=8.4/3.7ms, 1x1x1mm3); 3D DIR (TR/TE=5500/292ms, TI1/TI2= 525/2530ms, 1mm iso) and 3D FLAIR (TR/TE= 8000/375ms, TI=2356ms, 1x1x1mm3) for the segmentation of lesions using the semiautomatic JIM 8 software (Xinapse Systems, Essex, UK); 3D multi-echo GRE (TR/TE=55ms/5.2ms, flip angle=25°, 1x1x1mm3, 10 echoes) for the estimation of T2* relaxation time (mono-exponential fitting based on auto- regression on linear operations approach)3 ; 3D echo planar imaging Susceptibility weighted sequence (TR/TE=66/35ms, 0.67x0.67x0.67mm3) used to obtain both filtered phase (computed by applying Laplacian unwrapping and high pass gaussian filtering to the phase images)4 and Quantitative Susceptibility Mapping (QSM estimated using the Total Generalized Variation (TGV) algorithm)5; 3D GRE images images (TR/TE=25ms/3.7ms, flip angle=5°, 1x1x1mm3) acquired with and without the application of a magnetization transfer (MT) pulse and 3D T1w GRE (TR/TE=11ms/3.7ms, flip angle=15°, 1x1x1mm3) for the computation of both MTR and MTSat (an MT-based measure inherently compensated for B1 and T1 inhomogeneities)6; 3D Gradient and spin echo sequence (GRASE, TR/TE1/ΔTE=1056/10/10ms, flip angle=90°, 1x2x5mm3, 32 echoes) was used for the estimation of MWF (using multiexponential non linear least square fit)7.Filtered phase and QSM images allowed the classification of lesions as PRL+ (showing paramagnetic rim both on filtered phase and QSM) and PRL- (without paramagnetic rim in either filtered phase or QSM). An example of a lesion classified as PRL+ on both QSM and filtered phase is showed in figure 1 along with the corresponding MRI estimations. T2* maps, MTR, MTsat and MWF maps were registered to the SWI space and used in combination with the lesions masks obtained from the FLAIR images to evaluate the mean of the MRI parameters estimated in the core of each lesion (see figure 2 for the boxplots of the distribution of the MRI estimations obtained). The core of lesions was identified by 1-pixel erosion of the initial lesions masks.
Linear mixed effect models (LMEs) were used to compare between PRL+ and PRL- lesions the distributions of each estimated MRI parameter as well as lesions' volume while adjusting for confounding variables (i.e. age, sex and disease duration) and accounting for the subject-nested data.
RESULTS
987 lesions were identified, manually segmented and grouped according to the presence of the paramagnetic rim from a population of sixty-five MS patients (16 Males/49 Females, age mean ± standard deviation 36.1 ±10.4). 936 lesions were classified as PRL- lesions on both filtered phase and qsm images; 51 showed a paramagnetic rim on both filtered phase and qsm images.Statistically significant decrease in MTR (estimate=-3,867; p<0.001) and MTSat (estimate=-0,244; p<0.001) as well as increase in T2* (estimate=0,007; p=0.004), QSM (estimate=0,011; p<0.001) and lesions volume (estimate=1,52; p<0.001) was evidenced by the LMEs in PRL+ with respect to PRL-. LMEs did not reveal statistically significant changes when considering MWF.
DISCUSSION and CONCLUSION
In this study the MRI properties of PRL+ were investigated and compared to PRL-. The presence of a paramagnetic rim in susceptibility-based images in this study was associated with greater size as well as alterations in all the quantitative MRI parameters investigated except for MWF. The observed changes in MTR, MTsat, T2* and QSM were consistent with pathological alterations in macromolecules content and demyelination, however, the result obtained for MWF might have been affected by the lower voxel resolution and the GRASE type of readout. Nonetheless, the findings of this study support the hypothesis of an higher degree of demyelination and/or tissue damage characterizing PRL+ lesions with respect to PRL-, however, further studies on a larger patients cohort are necessary to increase the number of candidate PRL+ lesions. This MRI approach involving multiple MRI estimations has the potential to help assessing in-vivo the evolution and activity of MS lesions.Acknowledgements
No acknowledgement found.References
1. Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol (Berl). 2017;133(1):13-24. doi:10.1007/s00401-016-1653-y
2. Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol (Berl). 2017;133(1):25-42. doi:10.1007/s00401-016-1636-z
3. Pei M, Nguyen TD, Thimmappa ND, et al. Algorithm for fast monoexponential fitting based on Auto-Regression on Linear Operations (ARLO) of data. Magn Reson Med. 2015;73(2):843-850. doi:10.1002/mrm.25137
4. Absinta M, Sati P, Fechner A, Schindler MK, Nair G, Reich DS. Identification of Chronic Active Multiple Sclerosis Lesions on 3T MRI. Am J Neuroradiol. 2018;39(7):1233-1238. doi:10.3174/ajnr.a5660
5. Langkammer C, Bredies K, Poser BA, et al. Fast quantitative susceptibility mapping using 3D EPI and total generalized variation. NeuroImage. 2015;111:622-630. doi:10.1016/j.neuroimage.2015.02.041
6. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T 1 relaxation obtained from 3D FLASH MRI: Saturation and Relaxation in MT FLASH. Magn Reson Med. 2008;60(6):1396-1407. doi:10.1002/mrm.21732
7. Prasloski T, Rauscher A, MacKay AL, et al. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. NeuroImage. 2012;63(1):533-539. doi:10.1016/j.neuroimage.2012.06.064
Figures