2827
7-Tesla in vivo 1H-MRS-measured prefrontal glutathione increases during oral fumarate therapy in relapsing-remitting multiple sclerosis1Biomedical Engineering, Columbia University, New York, NY, United States, 2Radiology and Biomedical Imaging, Yale University School of Medicine, New Haven, CT, United States, 3Neurology, University of Southern California Keck School of Medicine, Los Angeles, CA, United States, 4Neurology, Yale University School of Medicine, New Haven, CT, United States, 5Radiology, Columbia University Irving Medical Center, New York, NY, United States
Synopsis
Keywords: Multiple Sclerosis, Spectroscopy, dimethyl fumarate, glutathione, oxidative stress
Multiple sclerosis (MS) is an autoimmune disease that damages the central nervous system. Oxidative stress, thought to play a role in MS-related pathophysiology, can be modulated in the cell by endogenous antioxidants such as glutathione (GSH), hypothesized to participate in the therapeutic effect of MS disease-modifying therapy dimethyl fumarate. We used proton magnetic resonance spectroscopy (1H MRS) to measure in vivo cortical glutathione concentrations in individuals with relapsing-remitting multiple sclerosis (RR-MS) before and during 12 months of dimethyl fumarate therapy and observed a significant positive effect of time on prefrontal cortex glutathione. No such change was shown in healthy controls.Introduction
Multiple sclerosis (MS) is a chronic autoimmune condition that damages cells in the central nervous system (CNS). Oxidative stress, or imbalance in cellular homeostatic redox potential, is thought to play a role in acute inflammation1 and neurodegeneration2,3, both seen in MS. Dimethyl fumarate is a methyl ester approved in 2013 by the United States Food and Drug Administration (USFDA) as an oral disease-modifying therapy (DMT) for the relapsing-remitting course of MS (RR-MS)4, which is characterized by intermittent periods of overt CNS inflammation and remissions thereof. Because dimethyl fumarate has been shown to increase the production of endogenous antioxidant glutathione in CNS cell cultures5 as well as reduce annual relapse rate relative to placebo in phase III studies of RR-MS treatment efficacy6,7, it is thought that the drug may ameliorate RR-MS-associated oxidative stress by supporting glutathione production, potentially via facilitating the nuclear translocation of nuclear transcription factor 2 (Nrf2) and its attendant modulation of genes related to glutathione synthesis8.In this single-arm open-label phase IV trial of oral dimethyl fumarate, we use in vivo proton magnetic resonance spectroscopy (1H MRS) to longitudinally measure cortical glutathione in a cohort of RR-MS patients at baseline and at multiple time points over one year following initiation of dimethyl fumarate treatment, as well as in a matched control group to assess methodological reproducibility.
Methods
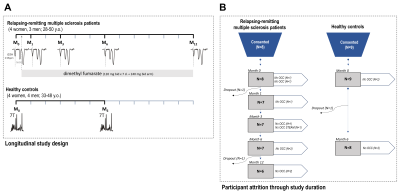
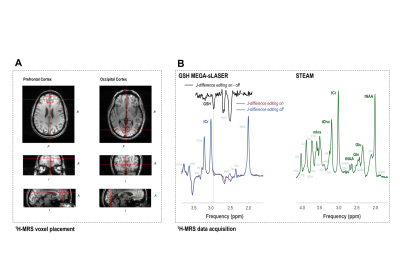
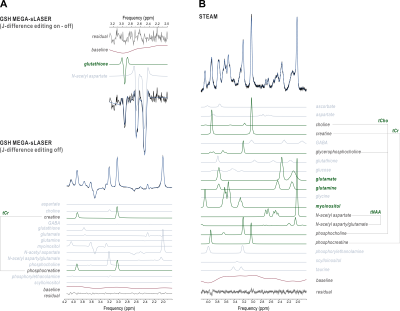
Seven RR-MS patients (4 female; 28-50 y.o.) were scanned according to a previously reported protocol9 on a 7-Tesla head-only magnet (Varian Medical Systems, Palo Alto, CA, USA) with an 8-channel transceiving radiofrequency coil at pre-treatment baseline and at one, three, six, and twelve months following initiation of oral dimethyl fumarate treatment (120 mg b.i.d. x 7 d., then 240 mg b.i.d.). Eight healthy controls (4 female; 33-48 y.o.) were also scanned at baseline and at Month 6 to assess reproducibility (Fig. 1). MEscher-GArwood semi-Localization by Adiabatic SElective Refocusing (MEGA-sLASER) for glutathione (TR 3 s; TE 72 ms; NA 64 per J-difference editing condition targeted to 4.56-ppm glutathione 7CH) and STimulated Echo Acquisition Mode (STEAM; TE 10 ms; TR 3 s; TM 50 ms; TI 320 ms; NA 96) for glutamate, glutamine, total N-acetyl aspartate, total choline, and myoinositol were acquired from 2.5 x 2.5 x 2.5 cm3 medial prefrontal and occipital cortex voxels (Fig. 2) manually positioned via T1-weighted imaging (200 x 220 x 78 mm3; 256 x 256 x 39 pts; TE 6 ms; TR 3 s) and quantified according to previously published methods10 for blind processing in INSPECTOR11,12 and linear combination modeling in LCModel13 (Fig. 3). All participants provided prior informed consent per approved IRB protocols.Metabolite concentrations were referenced to 10 mM total creatine. Generalized linear models with both numeric and factor coefficients time and random subject intercepts assessed metabolite concentration changes in each cohort. Statistics were calculated in R (v. 4.0.5) as means ± S.D; α = 0.05.
Results
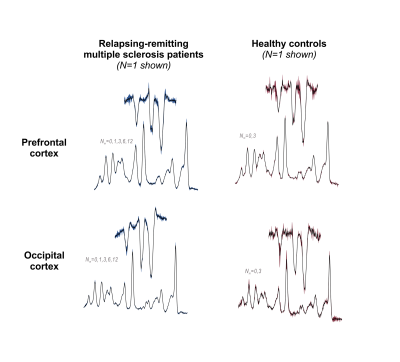
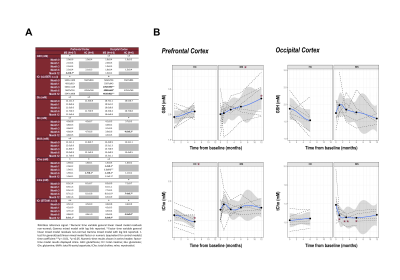
Visual inspection of acquired data affirmed acceptable spectral quality across time points (Fig. 4). In the RR-MS group only, prefrontal cortex glutathione demonstrated a significant positive effect of time as either a numeric (+0.05±0.02 mM/month, t(27)=2.6, p=0.02) or factor (+0.6±0.3 mM/12 months, t(24)=2.2, p=0.04) coefficient. No time effects on glutathione concentration were observed in either the occipital cortex or in the control group: Glutathione measurements demonstrated reproducibility across both time points in both voxels of the latter (Fig. 5).Among other metabolites measured by STEAM, prefrontal total choline (as factor: -0.08±0.02 mM/6 months, t(7)=-3.4, p=0.01), occipital glutamine (+0.3±0.07 mM/6 months, t(5)=4.0, p=0.01), and occipital myoinositol (+0.2±0.06 mM/6 months, t(5)=4.0, p=0.01) demonstrated changes across both time points in control, while reproducible control values were observed in all other metabolites and regions.
Conclusions
In this open-label, single-arm, single-center study on cortical glutathione measured by 1H MRS before and during oral dimethyl fumarate therapy in RR-MS patients, we have demonstrated the following:- Prefrontal cortex glutathione concentrations increased from 0 to 12 months in RR-MS patients treated with oral dimethyl fumarate;
- Occipital cortex glutathione concentrations did not significantly change in either the RR-MS or the control cohort during the study duration;
- While prefrontal cortex glutathione concentrations did not change during the study duration in the control group, multiple other metabolites including prefrontal total choline, occipital glutamine, and occipital myoinositol did, underlining the importance of replicating study results using expanded controls involving more time points and potentially a blinded placebo multiple sclerosis group.
Taken together, our findings provide preliminary evidentiary support for the previously hypothesized involvement of glutathione in the therapeutic benefit of dimethyl fumarate in relapsing-remitting multiple sclerosis, justifying the continued study of oxidative stress in the mechanisms of RR-MS and its amelioration by this and potentially also other DMTs.
Acknowledgements
We would like to thank all human participants for having volunteered their time and energy to this study as well as the physicians of the Yale-New Haven Hospital Interventional Immunology Clinic for patient referrals. This work was sponsored in part by Biogen Idec (Weston, MA) with additional support from NIH grants UL1 TR000142, R01 NS062885, and P30 NS052519, the National Multiple Sclerosis Society, and the Nancy Davis Foundation. This work was performed in part at the Zuckerman Mind Brain Behavior Institute MRI Platform, a shared resource and Columbia MR Research Center site.
References
1. Srinivasan, R., Ratiney, H., Hammond-Rosenbluth, K. E., Pelletier, D. & Nelson, S. J. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magnetic Resonance Imaging 28, 163-170 (2010).
2. Aoyama, K. & Nakaki, T. Impaired glutathione synthesis in neurodegeneration. International Journal of Molecular Sciences 14, 21021-44 (2013).
3. Bains, J. S. & Shaw, C. A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Research Reviews 25, 335-358 (1997).
4. United States Food and Drug Administration (USFDA), "FDA approves new multiple sclerosis treatment: Tecfidera," 2013.
5. Scannevin, R. H., Chollate, S., Jung, M. Y., Shackett, M., Patel, H., Bista, P., Zeng, W., Ryan, S., Yamamoto, M., Lukashev, M. & Rhodes, K. J. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. The Journal of Pharmacology and Experimental Therapeutics 341, 274-284 (2012).
6. Fox, R. J., Miller, D. H., Phillips, J. T., Hutchinson, M., Havrdova, E., Kita, M., Yang, M., Raghupathi, K., Novas, M., Sweetser, M. T., Viglietta, V. & Dawson, K. T. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. The New England Journal of Medicine 367, 1087-1097 (2012).
7. Gold, R., Kappos, L., Arnold, D. L., Bar-Or, A., Giovannoni, G., Selmaj, K., Tornatore, C., Sweetser, M. T., Yang, M., Sheikh, S. I. & Dawson, K. T. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. The New England Journal of Medicine 367, 1098-1107 (2012).
8. Bomprezzi, R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Therapeutic Advances in Neurological Disorders 8, 20-30 (2015).
9. Prinsen, H., de Graaf, R.A., Mason, G.F., Pelletier, D. and Juchem, C. "Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T," Journal of Magnetic Resonance Imaging 45, 187-198 (2017).
10. Swanberg, K. M., Prinsen, H., DeStefano, K., Bailey, M., Kurada, A. V., Pitt, D., Fulbright, R. K. & Juchem, C. In vivo evidence of differential frontal cortex metabolic abnormalities in progressive and relapsing-remitting multiple sclerosis. NMR in Biomedicine 34, e4590 (2021).
11. Gajdošík, M., Landheer, K., Swanberg, K. M. & Juchem, C. INSPECTOR: free software for magnetic resonance spectroscopy data inspection, processing, simulation and analysis. Scientific Reports 11, 2094 (2021).
12. Juchem, C. INSPECTOR - Magnetic Resonance Spectroscopy Software, <http://innovation.columbia.edu/technologies/cu17130_inspector> (2016).
13. Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine 30, 672-679 (1993).
Figures