2821
Identifying fiber tracts strategic for cognitive impairments in cerebral small vessel disease with harmonized diffusion MRI1Department of Radiology, Zheijang University School of Medicine,, Hangzhou, China, 2UMC Utrecht Brain Center, University Medical Center Utrecht, Utrecht, Netherlands, 3Aging and Cognition Center, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 4Institute for Stroke and Dementia Research, LMU Munich, Munich, Germany, 5Medical Image Analysis Center, University of Basel, Basel, Switzerland, 6Department of Geriatric Medicine, University Medical Center Utrecht, Utrecht, Netherlands, 7Division Neurology, The Chinese University of Hong Kong, Hong Kong, China, 8Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 9Division Imaging and Oncology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Keywords: Dementia, White Matter
Small vessel disease (SVD) is a worldwide leading cause of dementia. Damage to white matter tracts is a key mechanism through which SVD impacts cognition. In this work, we leverage a large multicenter dataset of patients with SVD to investigate which white matter tracts are strategic in SVD, that is, robustly associated with cognitive decline. Our early results show that the corpus callosum, superior longitudinal fasciculus and thalamo parietal tracts were the most associated to processing speed, verbal memory and executive function, respectively. Tract-based measures explained additional variance (2-5%) as compared to whole brain measures.Introduction
Cerebral small vessel disease (SVD) is related to half of dementia worldwide[1]. As SVD-related lesions mostly appear in the white matter, damage to fiber tracts is likely a key mechanism in determining the cognitive impact of SVD. In this work, we aim to identify fiber tracts robustly related to cognitive impairment (i.e., strategic) in a large international multi-center dataset.Methods
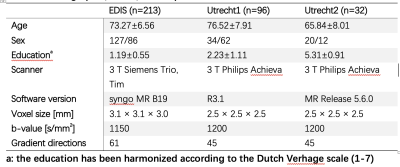
Individuals with sporadic SVD were screened from three previously published cohorts (EDIS, Utrecht1, and Utrecht2). EDIS is a community cohort for studying the epidemiology of dementia. The Utrecht1 cohort consisted of patients enrolled in a memory clinic, whereas the Utrecht2 cohort consisted of patients from a stroke clinic. Subjects were enrolled in the present study if they satisfied the following criteria: (1) with a Fazekas score≥2 or presence of lacune(s); (2) had T1, FLAIR, and diffusion MRI; (3) had cognitive data. Subjects with large stroke lesions or insufficient image quality were excluded.Three cognitive domains, attention and executive function (Att&EF), verbal memory (VMem), and processing speed(ProSpeed) were analyzed. A set of cognitive tests were assigned to each domain by a neuropsychologist. The z-scores of all tests within a specific domain were averaged to derive the final domain score[2].The imaging protocols of each center were reported[3], [4]. The rotation invariant spherical harmonic[5] (RISH) features were used to harmonize diffusion imaging data from different centers.
Deterministic tractography[6] was performed with MRIToolkit using the Generalized Richardson Lucy[7] approach. Subsequently, automatic clustering[8] was applied to identify 73 specific fiber tracts. We discarded cerebellar, superficial, and striatal tracts, and averaged bilateral fiber tracts as in a previous study[9]. This resulted in a total of 25 fiber tracts for each subject. The free water (FW) elimination model was applied using a script provided by the MarkVCID project (https://markvcid.partners.org/markvcid1-protocols-resources) to calculate FW and tissue FA (tFA). Fiber tract properties were extracted by applying the tractography masks in the native diffusion space. For statistical analyses, the associations between FW\tFA and each of the three cognitive domains were first investigated using simple linear regression in each cohort. Then we pooled all data and performed regression analyses adding the site information covariates in the regression model. For tract-wise analysis, the above procedures were repeated for each fiber tract. The adjusted R square was used to assess how much variance could be explained.
Results
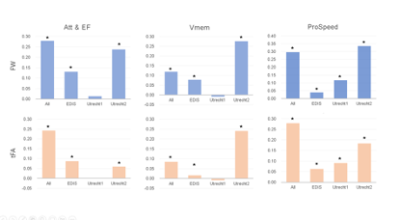
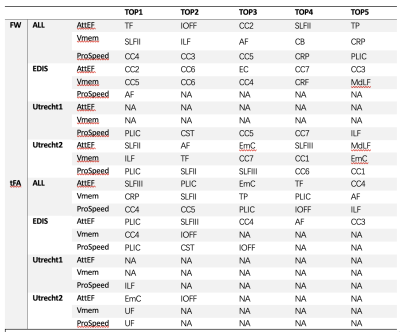
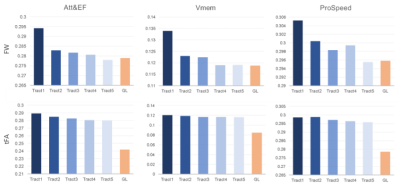
The study demographics and diffusion MRI acquisition settings are reported in Table 1. The Utrecht1 cohort had the highest global FW values, followed by Utrecht2 and EDIS. The tFA values showed the opposite trend. Compared to analyses in each individual cohort, the pooled analysis generally demonstrated more robust associations between diffusion metrics and cognitive measures (Figure 1).We listed the top 5 fiber tracts associated with each cognitive domain in the pooled analysis and each dataset in Table 2. As shown in the table, the pooled analysis yielded more significant fiber tracts than in the individual cohorts. Furthermore, tract-wise diffusion measures explained more variance compared to global measures in every cognitive domain (Figure 2).
Discussion and conclusion
We have identified fiber tracts that explain more variance in cognitive performance than whole brain measures. Strategic fiber tracts for Att&EF are mostly association fiber and cortical-subcortical connection fibers. For processing speed, the strategic fiber tracts mainly included the corpus callosum, the posterior limb of the internal capsule, and corona radiations. These fiber tracts are closely related to motor functions. For verbal memory, the arcuate fasciculus and several large association fiber tracts were crucial.In this preliminary analysis, tract-specific properties led to limited gains in explained variance, mostly between 2 and 5%, in line with other recent findings. This is partly unsurprising as cognitive decline is the result of complex interactions between fiber tracts, which might require more advanced analysis approaches to be better captured. Furthermore, we did not account for conventional lesion and neurodegeneration markers in this early phase, and their inclusion is likely to shade further light on the relation between tract-based measures and cognitive decline.
Acknowledgements
No acknowledgement found.References
[1] J. M. Wardlaw, C. Smith, and M. Dichgans, “Small vessel disease: mechanisms and clinical implications,” Lancet Neurol., vol. 18, no. 7, pp. 684–696, 2019.
[2] N. A. Weaver et al., “The Meta VCI Map consortium for meta-analyses on strategic lesion locations for vascular cognitive impairment using lesion-symptom mapping: Design and multicenter pilot study,” Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit., vol. 11, pp. 310–326, 2019.
[3] B. M. de Brito Robalo et al., “Diffusion MRI harmonization enables joint-analysis of multicentre data of patients with cerebral small vessel disease,” NeuroImage Clin., vol. 32, no. November, p. 102886, 2021.
[4] B. M. de Brito Robalo et al., “Improved Sensitivity and Precision in Multicentre Diffusion MRI Network Analysis Using Thresholding and Harmonization,” SSRN Electron. J., vol. 36, no. October, p. 103217, 2022.
[5] S. Cetin Karayumak et al., “Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters,” Neuroimage, vol. 184, no. September 2018, pp. 180–200, 2019.
[6] A. Leemans, B. Jeurissen, J. Sijbers, and D. K. Jones, “ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data.,” in 17th annual meeting of the International Society for Magnetic Resonance in Medicine, Honolulu, Hawaii, USA, 2009, p. 3537.
[7] F. Guo, A. Leemans, M. A. Viergever, F. Dell’Acqua, and A. De Luca, “Generalized Richardson-Lucy (GRL) for analyzing multi-shell diffusion MRI data,” Neuroimage, vol. 218, no. April, p. 116948, 2020.
[8] F. Zhang et al., “An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan,” Neuroimage, vol. 179, no. May, pp. 429–447, 2018.
[9] A. Dewenter et al., “Disentangling the effects of Alzheimer’s and small vessel disease on white matter fibre tracts,” Brain, pp. 1–35, Jul. 2022.
Figures