2817
Evaluation of relationship between trigeminal nerve stimulation and hemodynamics using 4D-Flow MRI: A pilot study in swine model1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 3Wisconsin Institute for Translational Neuroengineering (WITNe) – Madison, Madison, WI, United States, 4Neurosurgery, University of Wisconsin-Madison, Madison, WI, United States, 5Radiology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Keywords: Alzheimer's Disease, Velocity & Flow
This study utilizes 4D-Flow imaging to evaluate hemodynamic alterations during the period of nerve stimulation and investigates cerebral blood flow changes modulated by using 4D-Flow MRI in large animal models. Preliminary results showed cerebral blood flow increases while flow pulsatility decreases with the electrical stimulation of trigeminal nerves. This effect from neuromodulation is on the contrary to the hemodynamic alterations with aging and AD progression, which implies the potential of neuromodulation as an alternative treatment approach in repairing glymphatic system function.Purpose
While the exact causes and mechanisms of Alzheimer’s disease (AD) remain unclear, previous studies had shown an association between cerebrovascular disease (CVD) and AD development.[1] This may be due to the vascular disease itself negatively affecting brain health through decreased brain perfusion or end organ damage from adverse hemodynamic conditions (e.g., increased pulsatility, etc.); however, CVD has also been implicated in altered brain waste clearance through the glymphatic system. For this reason, the study of the cerebrovascular system and how the function of this system can be improved and/or manipulated has increased over recent years. It is well-established that electrical stimulation (Estim) thru trigeminal nerves can alter cerebral hemodynamics [2]. We hypothesize that such neuromodulation could repair glymphatic function that declines with age and delay disease progression, however, work is needed to validate the vascular and glymphatics effects of this mechanism. 4D-Flow imaging is a promising technique to provide flow measurements to better understand the effect of Estim. This work implements 4D-Flow imaging to observe hemodynamic alterations during the course of nerve stimulation and investigates CBF changes triggered by the non-invasive trigeminal nerve stimulation with large animal models using 4D-Flow MRI.Methods
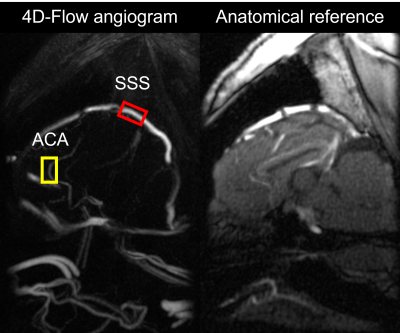
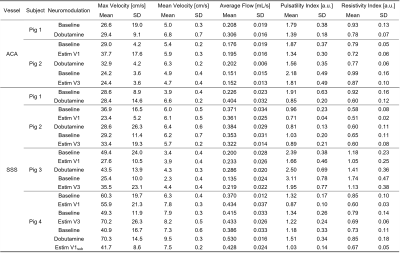
Neuromodulation protocol: A total of 4 mini pigs were imaged in this study. Subjects were anesthetized throughout the scanning session. Multiple baseline references were collected while no nerve stimulation (Estim) was performed. Two sets of Estim were instrumented via a noninvasive procedure using transcutaneous electrical nerve stimulation electrodes, targeting V1 and V3 branches of the trigeminal nerves, separately. An interleaved paradigm was used for Estim with 10-s onsets followed by 10-s rest periods during the entire image acquisition. Dobutamine was delivered as a positive control, which increases blood pressure level with limited effect on heart rate elevation. Contrast-enhanced 4D-Flow MRI: Prior to image acquisition, Ferumoxytol was administered to improve SNR at the dose of 4 mg Fe/kg as a slow infusion over 15 minutes, diluted by a normal saline continuous rate infusion. All subjects underwent MRI at a 3.0 T PET/MRI system (Signa PET/MR, GE Healthcare, WI) using a 32-channel head coil (Head 32 Flex, GE Healthcare, WI). A volumetric, time-resolved phase contrast (PC) MRI was collected using a 3D radially undersampled readout along with three-directional velocity encodings (PC-VIPR).[3] Relevant imaging parameters including TR/TE = 7.38/3.04 ms, flip angle = 15°, 5-point flow encoding scheme at Venc = 60 cm/s, field-of-view (FOV) = 240 x 240 x 240 mm3, number of projections = 16,000, total scan time ~10 mins. Image Reconstruction: Time-resolved data was obtained by retrospectively gating into 20 cardiac phases. Both time-averaged and time-resolved data were decoded and reconstructed into magnitude and velocity images (matrix size = 512 x 512 x 512) using PILS.[4] Flow analysis: Image analysis was performed by using a MATLAB-based program.[5] Quantitative metrics, including average flow and pulsatility index, were extracted from superior sagittal sinus (SSS) and anterior cerebral artery (ACA) for comparison (Figure 1). Pulsatility index was calculated from the cardiac-resolved flow measurements, defined as (Qmax-Qmin)/Qmean, where Qmax, Qmin, and Qmean refers to the maximum, minimum, and average flow across the time resolved estimates across cardiac cycle.Results
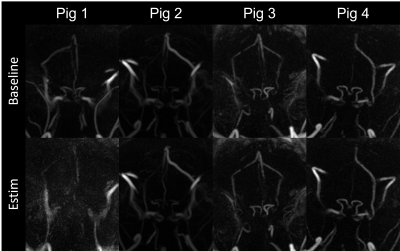
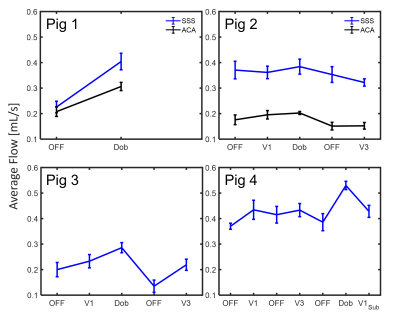
Figure 2 demonstrates maximum intensity projections generated from the 4D-Flow complex difference maps in each subject under baseline and Estim. Overall, the reconstructed pseudo-angiograms showed well-defined vasculature on the circle of Willis and sinus system. Yet, ACA was only seen and segmented on the first two pigs. During the initial trial on pig 1, Estim appears to have caused severe motion blurring. Subtle background noise was also found in pig 3 with inhomogeneity. Figure 3 shows average flow values for each individual subject. In general, average flow was found to be higher with the use of Dobutamine. Increased flow was found in with Estim compared to the baseline, but an opposite trend was also observed in the SSS segment of pig 2. Both pig 2 and 3 were found to have a declined flow value in the repeated baseline compared to its initial baseline level. A decreased flow pulsatility was also found in Estims compared to the baselines (Table 1).Discussion
This study successfully identified the hemodynamic alterations introduced by a non-invasive electrical stimulation scheme. It also revealed heterogeneous response to Estim in animals, indicating that optimization may be needed to alter vascular response. After pig 1, the magnitude of Estim was adjusted to prevent significant motion that would degrade image quality by visual assessment prior to the scans. Anesthesia and sub-motion threshold stimulation are limiting factors to such animal studies. To avoid a long lasting effect after dobutamine administration, a 10-min delay was introduced in between the scans. However, onset delay and/or washout effect of Estim has yet been well-reported. Temporal analysis, such as low-frequency flow oscillations and separation of onset/rest condition, can provide crucial information. Due to the nature of 3D radial acquisition, 4D-Flow is suitable for retrospectively reconstructed into high temporal resolution data to disentangle temporal information while the image quality permits.[6] In summary, this preliminary study provides an insight into neuromodulation from nerve stimulation into hemodynamics alternation and will inform the design of future animal and human subject studies.Acknowledgements
No acknowledgement found.References
[1] Vascular dysfunction-The disregarded partner of Alzheimer's disease. Sweeney MD, Montagne A, Sagare AP, et al. Alzheimers Dement. 2019;15:158-167.
[2] Trigeminal Nerve Control of Cerebral Blood Flow: A Brief Review.White TG, Powell K, Shah KA, Woo HH, Narayan RK, Li C.Front Neurosci. 2021 Apr 13;15:649910.
[3] Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Magn Reson Med. 2008 Dec;60(6):1329-36.
[4] Partially parallel imaging with localized sensitivities (PILS). Griswold MA, Jakob PM, Nittka M, Goldfarb JW, Haase A. Magn Reson Med. 2000 Oct;44(4):602-9.
[5] Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. Schrauben E, Wåhlin A, Ambarki K, Spaak E, Malm J, Wieben O, Eklund A. J Magn Reson Imaging. 2015 Nov;42(5):1458-64.
[6] Intracranial vascular flow oscillations in Alzheimer's disease from 4D flow MRI.Rivera-Rivera LA, Cody KA, Rutkowski D, Cary P, Eisenmenger L, Rowley HA, Carlsson CM, Johnson SC, Johnson KM. Neuroimage Clin. 2020;28:102379.
Figures