2815
Ultra-High Resolution QSM of the Brain Iron Metabolism in the Cognitively Declined Adults at 7T MRI1Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Biomedical Engineering, Boğaziçi University, Istanbul, Turkey, 3Department of Mechanical Engineering, University of Washington, Seattle, WA, United States, 4Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Keywords: Alzheimer's Disease, Quantitative Susceptibility mapping
Detection of iron aggregations associated with beta-amyloid in Alzheimer’s disease would help to understand the related pathophysiology in these cohorts. Aggregations of iron associated beta-amyloid should increase electron density and induce notable changes in local susceptibility value. With higher susceptibility at ultra-high field strengths, induced iron susceptibility is large enough to generate contrast relative to surrounding normal tissues that can be visualized by quantitative susceptibility techniques at 7 Tesla MRI. In this study, we used 7T MRI to analyze the iron-related pathologic markers in Alzheimer patients using the quantitative susceptibility mapping technique.Introduction
The extracellular aggregation of beta-amyloid (AB) plaques is one of the hallmark pathology of Alzheimer’s diseases (AD) that develops years prior to clinical symptoms [1]. However, inconsistency in the rate of cognitive decline among subjects with AB pathology and ineffectiveness of anti-amyloid therapeutics to mitigate cognitive impairment suggest that other parameters, such as patterns of brain iron, may impact the risk of cognitive decline. Quantitative susceptibility mapping (QSM) MRI techniques have enabled noninvasive measurement of tissue iron content at high spatial resolution and high sensitivity [2]. Prior QSM studies by several groups have reported increased cerebral iron in subcortical and cortical regions among subjects with AD and mild cognitive impairment (MCI) compared with healthy controls (HCs) [3,4]. Ultra-high field (UHF) MRI at 7 Tesla (7T) provides more than 2-fold signal-to-noise ratio (SNR) than 3T, and thus higher sensitivity and better MRI contrast [5]. In this study we leveraged improved SNR at 7T to explore susceptibility changes among individuals with AD/MCI and HCs through QSM with unprecedented resolution.Methods
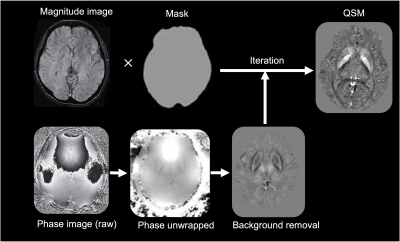
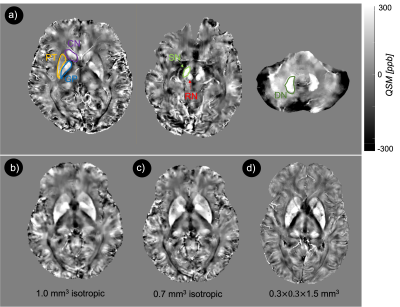
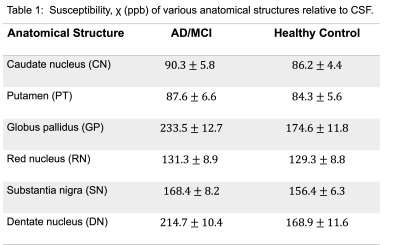
In vivo MRI experiment was performed in AD/MCI (N=4) and healthy subjects (N=4) aged 65-80 years and who satisfied the MR safety criteria. Informed consent was obtained from each subject under approval from the Institutional Review Board. Scanning was conducted on a 7T MRI scanner (Magnetom, Siemens Healthcare, Erlangen, Germany) using 1Tx/32Rx Nova head coil. To calculate QSM, a 3D high-resolution spoiled gradient echo (GRE) sequence was used (voxels size = 0.3x0.3x1.5 mm3, TR/TE = 32/10 ms, flip angle = 12 degree, and BW = 160 Hz/px). For one healthy subject three acquisitions were performed with identical parameters, except for the voxel size, which was 1.0 mm3 isotropic, 0.7 mm3 isotropic, and 0.3x0.3x1.5 mm3, respectively. Generation of QSM requires several discrete steps. First multi-echo phase images were combined supposing a linear weighted phase increment followed by Laplacian unwrapping [6]. Then, the background phase was removed using the variable-kernel sophisticated harmonic artifact reduction (VSHARP) for the phase data [7]. In the last step, QSM was calculated by dipolar inversion of the background corrected phase maps using the morphology enabled dipole inversion (MEDI) algorithm [8]. A brain mask was created by using the BET tool of FSL Software Library. The data processing chain for QSM is schematically illustrated in Fig. 1. QSM (Fig. 2d) was used to measure susceptibility in AD/MCI and health subjects among the major iron-enriched structures, including red nucleus (RN), substantia nigra (SN), globus pallidus (GP), Caudate nucleolus (CN), putamen (PT), and dentate nucleus (DN). We manually segmented the mentioned regions of interests (ROIs) based on non-susceptible anatomic reference sequences. The susceptibility values of the structures were normalized relative to the CSF.Results
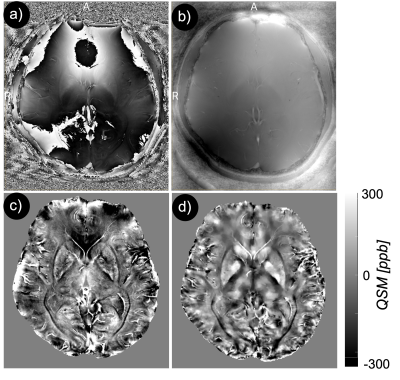
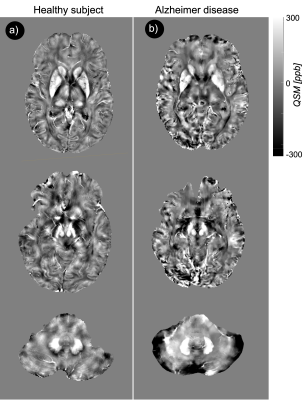
Fig. 2a showed the original phase image, from which the Fourier transform of the phase image was calculated. The Laplacian-based phase unwrapping allowed the visualization of the unwrapped phase (Fig. 2b). The background phase was then removed using VSHARP method (Fig. 2c). The MEDI method yielded high quality QSM without noticeable radial streaking artifacts (Fig. 2d). Figure 3a shows QSM images from different axial planes with corresponding ROIs. Comparing QSM with among pilot resolutions showed that 0.3x0.3x1.5 mm voxels offered an excellent contrast between gray and white matter which, allowed a clear brain structure delineation (Fig. 3b). We found a significantly higher magnetic susceptibility in GP and DN in patients with AD/MCI compared with HCs (Fig. 4). Overall mean susceptibility values for the RN, SN, CN, GP, PT, and DN in AD/MCI and HCs is summarized in Table 1. In AD/MCI the highest mean susceptibility values were found in the GP (233.5) and DN (214.7), verses 174.6 and 168.9 for GP and DN in the HCs, respectively. There were small differences in the susceptibility values between the other ROIs.Discussion and Conclusion
In this study, we reported an initial result for QSM at 7T in the limited cohorts of AD/MCI subjects and appropriate age matched HCs. The main aim of the study was to investigate whether susceptibility values measured using ultra-high resolution QSM differ in the iron-enriched regions between these two populations. A significant susceptibility value was reported in the AD/MCI compared with the HC in GP and DN regions. Overall, this difference is attribute to differences in iron accumulation and resultant magnetic effect. This is supported by data of other study groups which also found significant correlation between QSM and cognitive function [3,4]. Thus, this observation clearly deserves further investigations in larger cohorts.Acknowledgements
The authors would like to thank research coordinators Sarah Binder and Aislinn Diaz helping with the recruitment. This study was supported by a Developmental Project award from Mount Sinai ADRC (P30 AG066514) NIA/NIH and K01 AG075178-01 NIA/NIH grants.References
1. Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002 Oct 25;298(5594):789-91. doi: 10.1126/science.1074069. PMID: 12399581.
2. Liu C, Wei H, Gong NJ, Cronin M, Dibb R, Decker K. Quantitative Susceptibility Mapping: Contrast Mechanisms and Clinical Applications. Tomography. 2015 Sep;1(1):3-17. doi: 10.18383/j.tom.2015.00136. PMID: 26844301; PMCID: PMC4734903.
3. Chen L, Soldan A, Oishi K, Faria A, Zhu Y, Albert M, van Zijl PCM, Li X. Quantitative Susceptibility Mapping of Brain Iron and β-Amyloid in MRI and PET Relating to Cognitive Performance in Cognitively Normal Older Adults. Radiology. 2021 Feb;298(2):353-362. doi: 10.1148/radiol.2020201603. Epub 2020 Nov 24. PMID: 33231528; PMCID: PMC7850239.
4. Tiepolt S, Schäfer A, Rullmann M, Roggenhofer E; Netherlands Brain Bank, Gertz HJ, Schroeter ML, Patt M, Bazin PL, Jochimsen TH, Turner R, Sabri O, Barthel H. Quantitative Susceptibility Mapping of Amyloid-β Aggregates in Alzheimer's Disease with 7T MR. J Alzheimers Dis. 2018;64(2):393-404. doi: 10.3233/JAD-180118. PMID: 29865069.
5. Trattnig S, Springer E, Bogner W, Hangel G, Strasser B, Dymerska B, Cardoso PL, Robinson SD. Keyclinical benefits of neuroimaging at 7T. Neuroimage. 2018 Mar;168:477-489. doi: 10.1016/j.neuroimage.2016.11.031. Epub 2016 Nov 13. PMID: 27851995; PMCID: PMC5832016.
6. Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage. 2011 Apr 15;55(4):1645-56. doi: 10.1016/j.neuroimage.2010.11.088. Epub 2011 Jan9. PMID: 21224002; PMCID: PMC3062654.
7. Özbay PS, Deistung A, Feng X, Nanz D, Reichenbach JR, Schweser F. A comprehensive numerical analysis of background phase correction with V-SHARP. NMR Biomed. 2017 Apr;30(4):10.1002/nbm.3550. doi: 10.1002/nbm.3550. Epub 2016 Jun 3. PMID: 27259117; PMCID: PMC5136354.
8. Liu T, Liu J, de Rochefort L, Spincemaille P, Khalidov I, Ledoux JR, Wang Y. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: comparison with COSMOS in human brain imaging. Magn Reson Med. 2011 Sep;66(3):777-83. doi: 10.1002/mrm.22816. Epub 2011 Apr 4. PMID: 21465541.
Figures