2811
Validation Study of MRI Contrast Agent of Gd-DOTA Conjugated to DNA Aptamer for Detecting Oligomeric Amyloid-beta
Geon-Ho Jahng1, Sang-Tae Kim2, Hyug-Gi Kim3, Yu Mi Kim2, and Jee-Hyun Cho4
1Radiology, Kyung Hee University Hospital at Gangdong, Seoul, Korea, Republic of, 2Neuroscience of Lab, Seoul National University College of Medicine, Seongnam city, Korea, Republic of, 3Radiology, Kyung Hee University Hospital, Seoul, Korea, Republic of, 4Korea Basic Science Institute, Cheongju-si, Korea, Republic of
1Radiology, Kyung Hee University Hospital at Gangdong, Seoul, Korea, Republic of, 2Neuroscience of Lab, Seoul National University College of Medicine, Seongnam city, Korea, Republic of, 3Radiology, Kyung Hee University Hospital, Seoul, Korea, Republic of, 4Korea Basic Science Institute, Cheongju-si, Korea, Republic of
Synopsis
Keywords: Alzheimer's Disease, Molecular Imaging
The oligomeric amyloid-b (oAβ) is a reliable feature for an early diagnosis of Alzheimer's disease (AD). Therefore, the objective of this study was to demonstrate imaging of oAβ deposits using our developed DNA aptamer called ob5 conjugated with gadolinium (Gd)- DOTA as a contrast agent for early diagnosis of AD using MRI. An oAβ-specific aptamer was developed by amide bond formation and conjugated to Gd-DOTA MRI contrast agent and/or cyanine5 (cy5). We verified the performance of our new contrast agent with AD model mice using In vivo and ex vivo fluorescent imaging and animal MRI experiments.Introduction
Amyloid-beta (Aβ), one of the hallmarks of AD, shows an aberrant distribution in AD patients. The misfolding of mAβ precedes the formation of oligomers (1). Oligomeric forms of APP cleavage products play a pivotal role in the early stages of AD (2,3). Although MRI contrast agents have been developed to monitor amyloid plaques in animal brains (4,5,6,7,8,9), there is no MRI contrast agent available in the clinic for the detection of oAβ or Aβ plaque deposits in an AD patient. Aptamers can be rapidly acquired to identify sequences that can bind to a given target with high affinities using Selective Evolution of Ligands by Exponential enrichment (SELEX) (10,11). Currently available gadolinium (Gd)-based imaging agents can be easily conjugated into aptamers using labeled nucleotides, providing the potential for multiplexing and tuning reagents to the imaging platform. Therefore, the objective of this study was to demonstrate imaging of oAβ deposits using our developed DNA aptamer called ob5 conjugated with Gd-dodecane tetraacetic acid (DOTA) as a contrast agent for early diagnosis of AD using MRI. We verified the performance of our new contrast agent with AD model mice using In vivo and ex vivo fluorescent imaging and animal MRI experiments.Methods
DNA aptamer: The DNA aptamer called ob5 was constructed by amide bond formation for the detection of oAβ. A GdDOTA-ob5-cy5 contrast agent was constructed to evaluate its ability to detect oAβ using MRI and fluorescence imaging. oAβ binding sequence (ob5 aptamer) was linked to cy5 (cyanine5 flurophore; Bioneer, inc., Korea). GdDOTA-ob5-cy5 (eg, Gd-ob5-cy5) was newly synthesized as described previously (6,12, 13).MRI acquisition: All MRI experiments were performed on a 4.7 T animal MRI scanner (BioSpec 47/40; Bruker, Ettlingen, Germany). In vivo experiments and relaxivity measurement of GdDOTA-ob5 were performed as follows:
Relaxivity measurement of GdDOTA-ob5 contrast agent: Phantoms were developed at different GdDOTA-ob5 concentrations (mg/ml) to measure r1 and r2 relaxivities of GdDOTA-ob5 contrast agent with an animal 4.7 T MRI system. To measure r1 relaxivity, T1-weighted images were obtained for each phantom using a spin-echo sequence with the following parameters. To measure r2 relaxivity, T2-weighted images were also obtained for each phantom using the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence with keeping the same imaging parameters except the following parameters.
In vivo MRI experiment to measure MRI signal changes at specific brain areas: To evaluate MRI signal changes at specific brain areas after injecting the GdDOTA-ob5 contrast agent, T1-weighted images were acquired before and at nine minutes after injecting a commercial contrast agent (Dotarem®, Guerbet, France) or our contrast agent. Images were acquired for eight-month-old non-Tg mice injected with GdDOTA-ob5 and 3xTg AD mice injected with the commercial contrast agent at eight months old (n=3), with GdDOTA-ob5 at three months (n=3) and five months old (n = 3).
Repeated MRI scans after injection of the GdDOTA-ob5 contrast agent: To investigate MRI contrast changes after injecting the GdDOTA-ob5 contrast agent, T1-weighted images were acquired before and after injection of the GdDOTA-ob5 contrast agent. After injection of the contrast agent, T1-weighted images were repeatedly scanned at 5 min intervals until 30 minutes after injection for non-Tg mice (n=3) and 3xTg AD mice at 5 months (n=3) and 8 months old (n=3). Imaging parameters for T1-weighted MRI were the same as those used in the previous experiment.
Results
Validation experiments: Fig 1a shows aptamer molecular structure (A), concentration-response curve and calibration curve (insert) (B), and fluorescence spectra (C). Fig 1b shows the selectivity of the detection assay. Fig 1c shows the localization of oligomeric Aβ in caveolin/receptor-mediated endocytosis via bEND3 cells (A) and HT22 cells (B). Fig 1d shows confocal laser scanning microscopy images in bEND3 cells.Relaxivity measurement: A unique structural design in GdDOTA-ob5 contributed to its higher relaxivity. The structure of GdDOTA-ob5 as an MRI contrast agent substantially increased the rotational correlation time (τc).
In vivo MRI experiment to measure MRI signal changes at specific brain areas: Signal differences between before and after injection were larger with our contrast agent than with the commercial contrast agent (Dotarem® Gd-DOTA) for all three ROIs of 3-month-old and 5-month-old 3xTg AD mice (n = 3).
Repeated MRI scans after injection of the GdDOTA-ob5 contrast agent: For a 5-month-old 3x Tg AD mouse, MRI signals were increased in 5 minutes, prolonged until 10 minutes, and dropped at 15 minutes after injection of the contrast agent.
Conclusion
In conclusion, we demonstrated that the GdDOTA-ob5 contrast agent could be used as an MRI contrast agent to map oAβ in mouse brains. Our results suggest that the GdDOTA-ob5 contrast agent could be a useful MRI contrast agent for early detection of spreading toxic oAβ during the progression of AD. Future studies are needed to determine MRI metrics and quantitative changes of oAβ deposits in 3xTg-AD mice with enhanced AD-like neuropathology.Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare and Ministry of science and ICT, Republic of Korea (HU21C0086, G.H.J.).References
1. Huang, Y.R. and Liu, R.T., 2020. Journal of Lipid Research 58, 824-836. 2. Chae, C.W., et al., 2020.. Br J Pharmacol 177, 3828-3847. 3. Thomas, R.S., et al., 2016.. BMC Neurosci 17, 50. 4. Bort, G., et al., 2014.. Eur J Med Chem 87, 843-861 5. Dong, C.M., et al. Annu Int Conf IEEE Eng Med Biol Soc 2020, 1100-1103. 6. Dudeffant, C., et al., 2017. Sci Rep 7, 4955. 7. Luo, S., et al., 2020. Front Cell Neurosci 14, 21. 8. Patil, R., et al., 2015. Macromol Biosci 15, 1212-1217. 9. Zhang, D., et al., 2015.. Clin Radiol 70, 74-80. 10. Burke, D.H. and Gold, L., 1997. Nucleic Acids Res 25, 2020-2024 11. Horii, K., et al., 2010. Molecules 15, 5742-5755 12. Li, H. and Meade, T.J., 2019. J Am Chem Soc 141, 17025-17041. 13. So, M.K., et al., 2006. Nat Protoc 1, 1160-1164.Figures
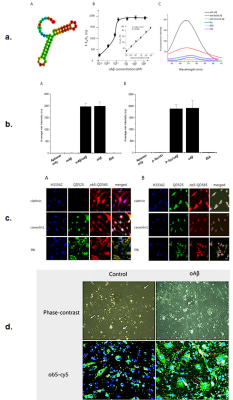
Figure
1. Results
of validation experiments.
Fig 1a shows
aptamer molecular structure (A), concentration-response curve and calibration curve (insert) (B), and
fluorescence spectra of the ob5 aptamer (C). Fig 1b shows the selectivity of the detection assay. Fig 1c shows the localization of oligomeric
Aβ in bEND3
cells (A) and in HT22 cells (B). Fig 1d shows confocal laser scanning microscopy images of classical
astrocyte cells in bEND3 cells.
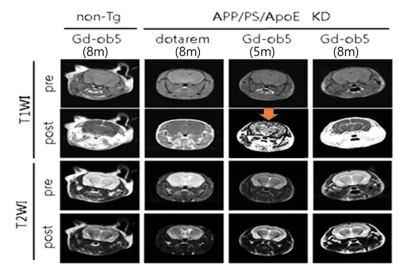
Figure
2. T1-weighted images (T1WI) and T2-weighted images (T2WI) before and after
injection of the GdDOTA-ob5 contrast agent or the commercial contrast agent
(Dotarem® Gd-DOTA) on the non-Tg and APP/PS/ApoE KD (3xTg) AD mice.
DOI: https://doi.org/10.58530/2023/2811