2798
Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) on variable diffusion times1Medical Imaging AI Research Center, Canon Medical Systems Korea, Seoul, Korea, Republic of, 2College of Medicine, Seoul National University, Seoul, Korea, Republic of, 3Magnetic Resonance Business Unit, Canon Medical Systems Korea, Seoul, Korea, Republic of
Synopsis
Keywords: White Matter, Diffusion/other diffusion imaging techniques
Diffusion tensor imaging (DTI) allows noninvasively to investigate the function of glymphatic system with ALPS index by utilizing the behavior of water molecules along the perivascular space. The effects of diffusion time with fixed TE for the evaluation of glymphatic system using diffusion tensor imaging have not been investigated. Here, we evaluated the effects of DTI with different diffusion time to ALPS index in the glymphatic system.Purpose
The glymphatic system was first discovered in a study in which mice were injected with an intrathecal fluorescent gadolinium-based contrast agent [1], It has since been found to play an important role in the pathophysiology of neurodegenerative diseases by being involved in the removal of waste products in the brain. However, administration of intrathecal contrast agent is an invasive method which can lead to serious neurotoxic complications and has not been approved for human in clinical. Alternatively, diffusion tensor imaging (DTI) allows noninvasively to investigate the function of glymphatic system with ALPS index by utilizing the behavior of water molecules along the perivascular space without the administration of contrast media [2]. ALPS index is derived as the ratio of motion of water molecules along the perivascular directions and the motion in the direction perpendicular to both major fiber tract (projection and associative fibers) and perivascular space. Previous studies suggested that ALPS index was directly correlated with clinical variables for Alzheimer’s disease, type 2 diabetic mellitus, idiopathic normal pressure hydrocephalus, mild cognitive impairment, dementia, and Parkinson’s disease [2-6]. However, the effects of diffusion time with fixed TE for the evaluation of glymphatic system using diffusion tensor imaging have not been investigated. Therefore, we evaluated the effects of DTI with different diffusion time to ALPS index in the glymphatic system.Materials & Methods
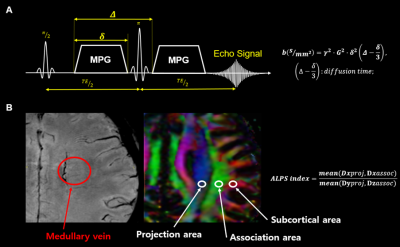
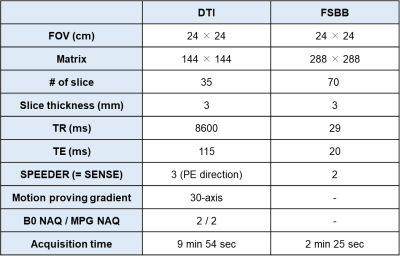
Flow Sensitive Black Blood (FSBB, to define the perivascular space) and SE-EPI-based DTI were acquired from 3 healthy subjects (male; 37~39 years old) using 3 T MRI scanner (Canon Medical Systems, Vintage Galan) with a 11-channel head and neck coil. The imaging parameters are summarized in Table 1. DTI sets with b = 1000 s/mm2 were acquired with diffusion time of 27, 34 and 45 ms with same TE. (Fig1.A).Diffusion metric images (Dx, Dy and Dz) were generated after correcting for distortion due to eddy currents and head motion using the FSL package. With identification of perivascular space based on the FSBB imaging, we defined the 3 ROIs for projection area, association area and subcortical area on the colored-FA map (Fig1.B). The diffusion metric data were extracted from these ROIs and then ALPS-index was calculated with the formula as shown in Fig1.B to assess the variation over the diffusion time.
Results
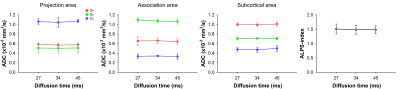
Figure 2 shows ADC value in three orthogonal directions for projection, association, and subcortical fibers as a function of diffusion time. The shorter diffusion times can induce the water diffusion less influenced by the structure, while longer times cause diffusion restricted by surrounding tissue. Although the diffusivities of z and x-axis in projection and subcortical fibers slightly increased with longer diffusion times, the overall patterns for the three directions (x, y, z) within the three areas (projection, association, subcortical) were independent of diffusion time. Accordingly, the ALPS-index was similar regardless of the variations of diffusion time (27 ms vs. 34 ms vs. 45 ms: 1.51 vs. 1.48 vs. 1.48).Discussion & Conclusion
Lee et al. reported the glymphatic activity to brain clearance was greater with sleep than wakefulness [7]. Another study demonstrated the significant correlation between glymphatic activity and aging. The DTI-ALPS method has been proposed to help assess the activity of the glymphatic system.Taoka et al. studied glymphatic function on DTI with different diffusion times of 29 ms, 35.7 ms, and 40.7 ms [8]. They demonstrated that shorter diffusion time (29 ms) resulted in significantly higher ALPS index compared with diffusion time of 35.7 ms and 40.7 ms with variable TE value ranged from 66 to 100 ms. In our study with small sample size, ALPS index increased somewhat with shorter diffusion time, but was not significantly affected by diffusion times varying from 27 ms to 45 ms at a fixed TE. The future study is required to investigate the effect of different diffusion time on glymphatic system in patients with neurodegenerative disease.
Acknowledgements
No acknowledgement found.References
1. Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018;3
2. Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol 2017;35:172-178
3. Steward CE, Venkatraman VK, Lui E, Malpas CB, Ellis KA, Cyarto EV, et al. Assessment of the DTI-ALPS Parameter Along the Perivascular Space in Older Adults at Risk of Dementia. J Neuroimaging 2021;31:569-578
4. Chen HL, Chen PC, Lu CH, Tsai NW, Yu CC, Chou KH, et al. Associations among Cognitive Functions, Plasma DNA, and Diffusion Tensor Image along the Perivascular Space (DTI-ALPS) in Patients with Parkinson's Disease. Oxid Med Cell Longev 2021;2021:4034509
5. Bae YJ, Choi BS, Kim JM, Choi JH, Cho SJ, Kim JH. Altered glymphatic system in idiopathic normal pressure hydrocephalus. Parkinsonism Relat Disord 2021;82:56-60
6. Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage 2021;238:118257
7. Lee S, Yoo RE, Choi SH, Oh SH, Ji S, Lee J, et al. Contrast-enhanced MRI T1 Mapping for Quantitative Evaluation of Putative Dynamic Glymphatic Activity in the Human Brain in Sleep-Wake States. Radiology 2021;300:661-668
8. Taoka T, Ito R, Nakamichi R, Kamagata K, Sakai M, Kawai H, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol 2022;40:147-158
Figures