2792
The value of MR diffusion tensor imaging in assessing white matter changes in short-term methamphetamine withdrawal1The Second Xiangya Hospital, Central South University, Changsha, China, 2MR Application,Siemens Healthineers Ltd., Changsha, China, 3MR Scientific Marketing,Siemens Healthineers Ltd., Wuhan, China
Synopsis
Keywords: White Matter, Diffusion/other diffusion imaging techniques
This study aimed to explore the changes of brain white matter changes in short-term methamphetamine (MA) abstinence and to investigate potential imaging markers. Compared with healthy controls (HC), fractional anisotropy (FA), axial diffusivty (AD) and mean diffusivity (MD) values in short-term abstinence group were all increased, but there was no significant difference in the radial diffusivty value. The changes of FA, AD and MD value may be a new biomarker which is helpful to explore the potential mechanism of neurotoxicity damage.Introduction
In 2019 more than 27 million people have used amphetamine-type stimulants in the past year, with increasing use of methamphetamine in particular1. Although studies have suggested that MA abuse may cause structural and functional changes in the brain, the small sample size across studies due to subject specificity makes the results of discrepant imaging metrics controversial; and most current studies have explored changes in brain structure after long-term withdrawal from MA abuse2. Diffusion tensor imaging (DTI) can noninvasively reflect brain tissue's structural integrity and physiological state by measuring the diffusion process of water molecules3, 4. Therefore, the present study proposes to use DTI tract-based spatial statistics (TBSS) analysis to compare the alterations in white matter microstructure between the brains of 55 MA short-term abstainers and 52 sex-, age-, and education-matched healthy controls (HC), with the aim of exploring the underlying mechanisms of neuropsychiatric symptoms in short-term abstinent MA abusers.Methods
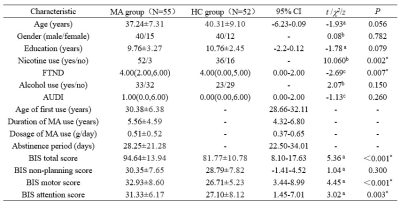
Prospective information was collected from 55 MA short-term abstainers and 52 HC sites from August 2017 to December 2018. All subjects underwent MRI and their demographic information was collected, including age, gender, education, age at first MA use, duration of MA use, the daily dose used, duration of withdrawal, the Fagerstrom Test for Nicotine Dependence (FTND), the Alcohol Use Disorders Screening Scale (AUDIT), and the Barratt Impulsivity Scale-11 (BIS-11), the eleventh edition of the Alcohol Use Disorders Identification Test (AUDIT). All MRI data were acquired on a 3.0T German Siemens Skyra MRI scanner equipped with a 32-channel head coil. DTI was acquired using the following parameters: b=1,000 mm/s2 and b=0 mm/s2 for a total of 64 gradient directions, TR=9,500ms, TE=88ms, number of layers=60, layer thickness=2mm, layer spacing=0mm, scan field=256×256mm2, voxel size=2×2×2mm3. image processing included preprocessing and diffusion index calculation. The software used for data processing was the FMRIB software library (FSL, http: //fs1.fmrib.ox.ac.uk/fsl/fslwiki/). Pearson correlation analysis was used to perform correlation analysis.Results
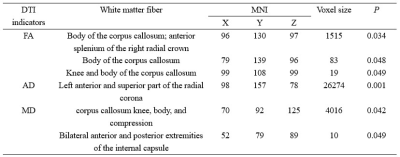
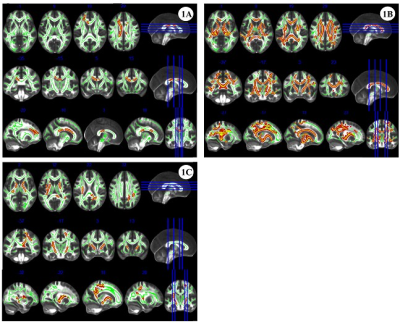
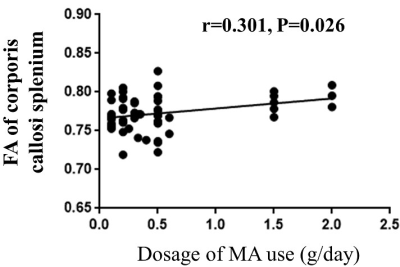
The comparison of demographic and behavioral scales between the two groups is shown in Table 1. FA, AD, and MD values were elevated in the MA group with short-term withdrawal compared to the HC group, while the difference in RD values between the two groups was not statistically significant (Figure 1). Detailed information on the areas with differences in the above 3 indices, including the name of the specific affected white matter bundle, voxel size, and MNI coordinates are listed in Table 2. FA values in the corpus callosum that differed between the MA and HC groups were positively correlated with the daily dose of MA use (r = 0.301, P = 0.026, PFWE = 0.625) (Figure 2).Discussion
In this study, FA, AD, and MD values were elevated in the knee and body of the corpus callosum, the anterior part of the bilateral corona radiata and the superior part of the left corona radiata; the knee, body, and pressure of the corpus callosum, the anterior limb of the internal capsule, the posterior limb of the internal capsule, the anterior, superior, and posterior parts of the corona radiata, the external capsule, and the superior longitudinal fasciculus; and the superior longitudinal fasciculus, the anterior limb of the internal capsule, and the posterior limb of the internal capsule on the right. The main role of the corpus callosum is to connect the two sides of the brain, which is the "essential pathway" for information transfer between the two hemispheres, and several studies have shown that substance abuse can lead to a decrease in the integrity of white matter fibers in the knee of the corpus callosum2, and that the superior longitudinal tract connects the frontal and parietal lobes, and the above white matter damage may also affect to some extent the functions related to the prefrontal lobe control, memory and other functions associated with the prefrontal lobes5, but unfortunately no correlation between changes in corpus callosum or prefrontal DTI indicators and the BIS-11 impulse-type scale has been found in the present study; the radial corona is a radial white matter fiber with an as yet unclear arrangement pattern from the internal capsule to the cerebral cortex, the anterior limb of the internal capsule separating the caudate and ducal nuclei, the posterior limb of the internal capsule separating the thalamus and ducal nuclei, and the external capsule separating the ducal and pallid nuclei. These structures play an important role in the thalamocortical and cortico-striato-thalamo-cortical loops, which are the basic neural network for behavioral changes in the brain6; and, studies related to the damage to the white matter fibers in and around the internal capsule by addictive disorders have been widely reported, such as alcohol addiction and cocaine addiction7, 8.Conclusion
In conclusion, there was white matter fiber edema and damage in MA abusers with short-term withdrawal, and the degree of damage to the corpus callosum was positively correlated with the daily dose of MA use. This finding contributes to the understanding of the pathophysiological process of neurological damage and the underlying mechanisms of neuropsychiatric symptoms in MA abusers with short-term withdrawal.Acknowledgements
No acknowledgement found.References
[1] World Drug Report. https://www.unodc.org/unodc/data-and-analysis/wdr2021.html. (2021) [2022-01-13].
[2] Huang S, Yang W, Luo J, et al. White Matter Abnormalities Based on TBSS and Its Correlation With Impulsivity Behavior of Methamphetamine Addicts[J]. Front Psychiatry, 2020,11:452. DOI: 10.3389/fpsyt.2020.00452.
[3] W Y, S W, Z S, et al. Novel circuit biomarker of impulsivity and craving in male heroin-dependent individuals[J]. Drug Alcohol Depen, 2021,219:108485. DOI: 10.1016/j.drugalcdep.2020.108485.
[4] Beard C L, Schmitz J M, Soder H E, et al. Regional differences in white matter integrity in stimulant use disorders: A meta-analysis of diffusion tensor imaging studies[J]. Drug Alcohol Depen, 2019,201:29-37. DOI: 10.1016/j.drugalcdep.2019.03.023.
[5] You S S, Chang C H, Jung Y J, et al. Injury of the Oculomotor Nerve After Aneurysmal Subarachnoid Hemorrhage: Diffusion Tensor Tractography Study[J]. Am J Phys Med Rehabil, 2015,94(6). DOI: 10.1097/PHM.0000000000000270.
[6] Kopell B H, Greenberg B D. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry[J]. Neurosci Biobehav R, 2008,32(3):408-422. DOI: 10.1016/j.neubiorev.2007.07.004. [7] Yeh P H, Simpson K, Durazzo T C, et al. Tract-based spatial statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: Abnormalities of the motivational neurocircuitry[J]. Psychiatry Res, 2009,173(1):22-30. DOI: 10.1016/j.pscychresns.2008.07.012.
[8] Bell R P, Foxe J J, Nierenberg J, et al. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals[J]. Drug Alcohol Depen, 2011,114(2-3):159-168. DOI: 10.1016/j.drugalcdep.2010.10.001.
Figures