2780
Feasibility study of synthetic MRI in shoulder imaging1Ningxia Medical University General Hospital Radiology department, Yinchuan, China
Synopsis
Keywords: Multimodal, Tendon/Ligament
In this work,we found that the image quality of shoulder synthetic MRI sequence is not comparable to that of conventional image quality, and the scanning time is long, but once scanning can generate multiple sets of quantitative images, T2 mapping of synthetic sequence can provide a new quantitative method for the diagnosis and differential diagnosis of supraspinatus tendon related diseases.Introduction
Synthetic MRI is a novel multimodal quantitative MRI technique that can generate multiple sets of conventional weighted sequences and quantitative sequences at one time, shorten the scanning time, reflect the quantitative information of human tissue components, and improve the feasibility of clinical application [1,2]. Synthetic MRI can generate different image contrasts by adjusting TE and TR generated inherent T1 relaxation, T2 relaxation and proton density[1,3], which are absolute magnetic resonance properties of any living tissue[4], achieve quantitative assessment of early biochemical changes in various tissues such as collagen fiber structure, orientation and water content [5-7]. Currently, synthetic MRI are clinically applied to the central nervous system, breast and prostate in addition to the central nervous system [8-11], and many studies have confirmed its feasibility in imaging the temporomandibular joint, lumbar intervertebral disc lesions and sacroiliac joints [12-14] and other bony muscle systems, Yi et al.[13] and Fayad et al.[14] also reported the feasibility of synthetic MRI in knee imaging. However, the clinical application of synthetic MRI in assessing the rotator cuff injury is still little understood.Therefore, In this work, the feasibility of magnetic resonance image compilation in volunteers shoulder scanning were investigated.
Methods and Materials
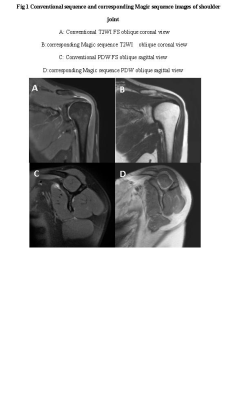
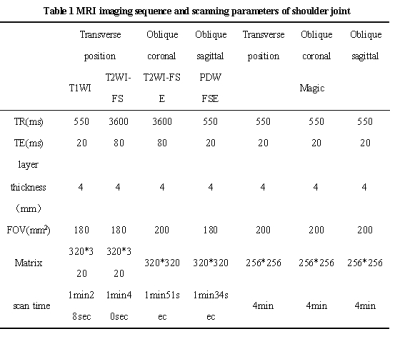
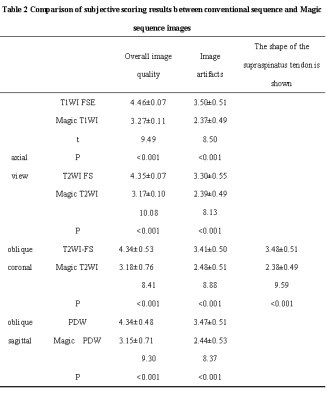
1.This study was approved by the Institutional Review Board and all subjects provided informed consent.2.67 volunteers were prospectively enrolled for shoulder MRI conventional sequence (axial view T1WI FSE and T2WI FS, oblique coronal T2WI FS and oblique sagittal PDW) and MRI compilation sequence (axial, oblique coronal and oblique sagittal view).The conventional and Magic sequences were scored subjectively by two researchers in terms of overall image quality, artifacts and the sharpness of the supraspinatus tendon.
3.The signal intensity of humeral cephalomedullary cavity and deltoid muscle, standard deviation of air was measured on conventional sequences ,the T1, T2 and PD values of the humerus head medullary cavity and deltoid muscle were measured by Magic T1,T2 and PD mapping image.The signal noise ratio (SNR) and carrier to noise ratio (CNR) were calculated.
4.Paired t-test was used to compare the objective indexes of conventional images and Magic images, rank test was used to compare the subjective scores of images, and Kappa test was used to evaluate the consistency of subjective scores between two doctors.
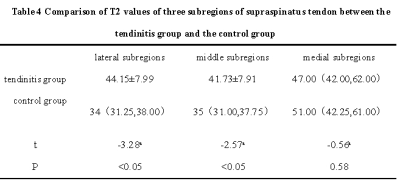
5.Another 41 patients with tendinitis diagnosed by clinical and imaging data were included.The supraspinatus tendon was divided into lateral, medial and middle subregions according to its shape in oblique coronal T2WI view on Magic sequence.The T2 values of the supraspinatus tendon on the Magic oblique coronal images were measured .One-way analysis of variance were used to compare the differences of T2 values in different regions. Receiver operating characteristic (ROC) curves were drawn with statistically significant differences to evaluate the diagnostic efficacy for tendinitis.
Results
1. The overall image quality subjective scores and artifacts scores of Magic sequences in axial, oblique coronal and oblique sagittal images were lower than those of conventional T1WI,T2WI FS and PDW sequences, and the differences were statistically significant (P < 0.001). The scores of supraspinatus tendon sharpness in Magic oblique coronal T2WI view was significantly lower than conventional oblique coronal T2WI FS image, and the difference was statistically significant (t =9.59,P < 0.001).2. There was no significant difference in SNR between conventional T1WI and Magic T1WI sequence (t = -1.351, P>0.001), but there were significant differences in SNR and CNR between other conventional sequences and corresponding Magic sequence (all P < 0.001).
3. The T2 values of lateral subregion and middle subregion in the tendonitis group were (44.15±7.99,41.73±7.91) ,higher than those in the control group (37.04±7.38,37.17±6.89), and the differences were statistically significant (P<0.001). There was no significant difference in the results of medial subregion (all P >0.001).
4. The AUC of T2 value in the lateral subregion was 0.733, and the AUC of T2 value in the middle subregion was 0.682. The above two groups of values had high diagnostic efficacy for supraspinatus tendinitis.
Conclusions
The image quality of shoulder synthetic MRI sequence is not comparable to that of conventional image quality, and the scanning time is long, but once scanning can generate multiple sets of quantitative images, T2 mapping of synthetic sequence can provide a new quantitative method for the diagnosis and differential diagnosis of supraspinatus tendon related diseases.Discussion
In this work, some differences were nevertheless observed between the conventional sequence and Magic sequence view. The synthetic sequence, on occasion, had less contrast, a “dirty” appearance to the images, and less resolution, resulting in lower image-quality scores assigned by the readers in some cases[15-18]. These differences were statistically significant and affect the overall perception ofimaging quality or the diagnostic confidence. Therefore,conventional sequences remain the first choice in the assessment of shoulder disease.However, synthetic MR imaging once scanning can generate multiple sets of quantitative images, T2 mapping can provide a new quantitative method for the diagnosis and differential diagnosis of supraspinatus tendon related diseases.
Acknowledgements
No acknowledgement found.References
[1] Drake-Perez M, Delattre B, Boto J, et al. Normal Values of Magnetic Relaxation Parameters of Spine Components with the Synthetic MRI Sequence[J]. AJNR Am J Neuroradiol, 2018,39(4):788-795.DOI:10.3174/ajnr.A5566.
[2] Lou B, Jiang Y, Li C, et al. Quantitative Analysis of Synthetic Magnetic Resonance Imaging in Alzheimer's Disease[J]. Front Aging Neurosci, 2021,13:638731.DOI:10.3389/fnagi.2021.638731.
[3] Schmidbauer V, Geisl G, Diogo M, et al. SyMRI detects delayed myelination in preterm neonates[J]. Eur Radiol, 2019,29(12):7063-7072.DOI:10.1007/s00330-019-06325-2.
[4] Goncalves F G, Serai S D, Zuccoli G. Synthetic Brain MRI: Review of Current Concepts and Future Directions[J]. Top Magn Reson Imaging, 2018,27(6):387-393.DOI:10.1097/RMR.0000000000000189.
[5] Roux M, Hilbert T, Hussami M, et al. MRI T2 Mapping of the Knee Providing Synthetic Morphologic Images: Comparison to Conventional Turbo Spin-Echo MRI[J]. Radiology, 2019,293(3):620-630.DOI:10.1148/radiol.2019182843.
[6] Schmidbauer V, Dovjak G, Geisl G, et al. Impact of Prematurity on the Tissue Properties of the Neonatal Brain Stem: A Quantitative MR Approach[J]. AJNR Am J Neuroradiol, 2021,42(3):581-589.DOI:10.3174/ajnr.A6945.
[7] Zhao L, Liang M, Shi Z, et al. Preoperative volumetric synthetic magnetic resonance imaging of the primary tumor for a more accurate prediction of lymph node metastasis in rectal cancer[J]. Quant Imaging Med Surg, 2021,11(5):1805-1816.DOI:10.21037/qims-20-659.
[8] Ambarki K, Lindqvist T, Wahlin A, et al. Evaluation of automatic measurement of the intracranial volume based on quantitative MR imaging[J]. AJNR Am J Neuroradiol, 2012,33(10):1951-1956.DOI:10.3174/ajnr.A3067.
[9] Jung Y, Gho S M, Back S N, et al. The feasibility of synthetic MRI in breast cancer patients: comparison of T2 relaxation time with multiecho spin echo T2 mapping method[J]. Br J Radiol, 2018:20180479.DOI:10.1259/bjr.20180479.
[10] Arita Y, Takahara T, Yoshida S, et al. Quantitative Assessment of Bone Metastasis in Prostate Cancer Using Synthetic Magnetic Resonance Imaging[J]. Invest Radiol, 2019,54(10):638-644.DOI:10.1097/RLI.0000000000000579.
[11] Lee C, Choi Y J, Jeon K J, et al. Synthetic magnetic resonance imaging for quantitative parameter evaluation of temporomandibular joint disorders[J]. Dentomaxillofac Radiol, 2021,50(5):20200584.DOI:10.1259/dmfr.20200584.
[12] Jiang Y, Yu L, Luo X, et al. Quantitative synthetic MRI for evaluation of the lumbar intervertebral disk degeneration in patients with chronic low back pain[J]. Eur J Radiol, 2020,124:108858.DOI:10.1016/j.ejrad.2020.108858.
[13] Yi J, Lee Y H, Song H T, et al. Clinical Feasibility of Synthetic Magnetic Resonance Imaging in the Diagnosis of Internal Derangements of the Knee[J]. Korean J Radiol, 2018,19(2):311-319.DOI:10.3348/kjr.2018.19.2.311.
[14] Fayad L M, Parekh V S, de Castro L R, et al. A Deep Learning System for Synthetic Knee Magnetic Resonance Imaging: Is Artificial Intelligence-Based Fat-Suppressed Imaging Feasible?[J]. Invest Radiol, 2021,56(6):357-368.DOI:10.1097/RLI.0000000000000751.
[15] Blystad I, Warntjes JB, SmedbyO, et al. Synthetic MRI ofthe brain in a clinical setting. Acta Radiol 2012;53:1158–63 CrossRefMedline.
[16] Lee SM, Choi YH, Cheon JE, et al. Image quality at synthetic brain magnetic resonance imaging in children. Pediatr Radiol 2017;47: 1638–47 CrossRefMedline.
[17] Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for clinical neuroimaging: results of the Magnetic Resonance Image Compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am J Neuroradiol 2017;38:1103–10 CrossRefMedline.
[18] Granberg T, Uppman M, Hashim F, et al. Clinical feasibility of synthetic MRI in multiple sclerosis: a diagnostic and volumetric validation study. AJNR Am J Neuroradiol 2016;37:1023–29 CrossRef Medline.
Figures