2761
A novel approach to derive robust arterial input functions for DCE-MRI in small animals1Department of Infection, Immunity and Cardiovascular Disease, The University of Sheffield, Sheffield, United Kingdom, 2Institute of Medical Physics, The University of Sydney, Sydney, Australia, 3Centre for Imaging Sciences, Division of Informatics Imaging & Data Sciences, School of Health Sciences, Faculty of Biology Medicine & Health, University of Manchester, Manchester, United Kingdom, 4Bioxydyn Ltd, Manchester Science Park, Manchester, United Kingdom, 5BioVentureHub, Antaros Medical, Mölndal, Sweden, 6MedTech West, Chalmers University of Technology, Gothenburg, Sweden, 7MR & CT Contrast Media Research, Bayer AG, Berlin, Germany
Synopsis
Keywords: Validation, Animals, Arterial input functions, small animals, spleen, liver
Accurate biomarker quantification is hindered in small animal MRI due to difficulties in reliably deriving arterial input functions (AIFs). This study provides a robust alternative to commonly used approaches by deriving AIFs from a simple, whole-body circulation model. This method is compared with individual and population spleen-derived AIFs by evaluating performance in gadoxetate DCE-MRI of the rat liver. Results demonstrated that the whole-body circulation model-derived AIF yields greater repeatability, reproducibility, and goodness-of-fit to observed data, indicating that it provides more accurate biomarker quantification than the individual or population spleen-derived AIFs.
INTRODUCTION
Robust arterial input functions (AIFs) are critical for accurate quantification in dynamic contrast-enhanced MRI (DCE-MRI), yet their measurement is typically challenging in small animals due to the risk of partial volume effects in the arteries.1 Consequently, studies either derive AIFs from tissue concentrations in reference organs, or use fixed population-averages measured separately.2 In this abstract, we propose a novel method for deriving AIFs based on a whole-body circulation model. We compare this with both individual and population spleen-derived AIFs for gadoxetate DCE-MRI of the liver in 90 rat datasets.METHODS
Model-based AIFA schematic representation of the whole-body circulation model-derived AIF used in this study is shown in Fig. 1. Two compartments are sufficient in rats due to rapid contrast agent circulation mixing relative to typical injection times. The model uses only four physiological parameters which can either be fitted from measured data, or, when using well-characterised animal models, can be set to literature values derived from pharmacokinetic studies. In this study the values were fixed to literature data provided in Table 1.
Population
Data was collected retrospectively from two multi-centre studies administering saline (control) or a compound of interest (treatment) to rats prior to baseline and follow-up acquisitions:
1) Test-retest study
Nrats=13. Baseline=saline; follow-up=saline.
2) Six compounds study
Nrats=32. Baseline=saline; follow-up=Asunaprevir, Bosentan, Ciclosporin, Ketoconazole, Pioglitazone, or Rifampicin.
MRI protocol
MRI was performed using two 4.7T and two 7T Bruker (Ettlingen, Germany) scanners using a T2-weighted (T2W) spin echo sequence for anatomy identification and a retrospectively triggered 3D Fast Low Angle Shot (FLASH) RF-spoiled gradient echo T1W acquisition (TE/TR=1.1/5.8 ms; Nslices=26; slice thickness=1.35 mm; FOV=60 x 60 x 35 mm; matrix size=64 x 64 x 26; resolution=0.94 x 0.94 x 1.35 mm).
MRI processing
Spleen MRI signals were used to derive individual AIFs, which were subsequently pooled together over all rats for the population-derived AIF. Using the model outlined in Sourbron et al.3, rate constants for hepatic plasma clearance (Ktrans) and uptake (khe), and biliary efflux (kbh) were calculated via tracer kinetic modelling.
Statistical analysis
1) Repeatability and reproducibility
From the test-retest study, within-subject repeatability and between-subject reproducibility relative errors (RE; %) for Ktrans, khe, and kbh were calculated for each AIF choice. Statistical significance (p<0.05) was assessed via a one-way ANOVA.
2) Goodness-of-fit
From the six compounds study, Root Mean Squared Percentage Errors (RMSPEs) and Akaike Information Criterion (AIC) scores were used to determine goodness-of-fit of observed to fitted liver signals. AIC score probabilities and evidence ratios were used to determine the relative likelihood of AIF choices. An AIF was deemed to have significantly greater relative likelihood in cases where both an AIC score probability > 99% and evidence ratio > 100 was observed.
RESULTS
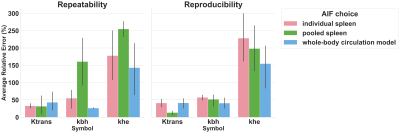
Randomly selected example fits are shown in Fig. 2, below. Generally, the whole-body model fits the data well and is at least as good as the alternatives. Physiologically implausible (exponential) khe values were estimated in 50% of cases for the pooled spleen AIF, compared with 36% and 8% for the individual spleen and whole-body circulation model AIFs, respectively.Test-retest study
The whole-body circulation model exhibited best repeatability for kbh (p = 0.008), however differences for Ktrans and khe were not significant. Conversely, best reproducibility was demonstrated by the pooled spleen AIF for Ktrans (p = 0.03), with no significant differences observed for kbh and khe.
Six compound study
Average RMSPEs and AIC scores are shown in Table 2. The individual spleen and whole-body circulation model were found to have significantly greater relative likelihood with respect to the pooled spleen in 61% and 47% of cases, respectively. In 33% of cases, the individual spleen was found to have a significantly greater relative likelihood with respect to the whole-body circulation model.
DISCUSSION
The whole-body circulation model was associated with lowest RMSPEs and AIC scores, however, did not demonstrate significantly greater REs across biomarkers. In cases where the gadoxetate extraction fraction, E approaches 100%, derived Ktrans values approach the fixed liver perfusion rate (Fp=1.32114 mL/min/mL), consequently affecting estimated khe values derived from Ktrans. In such cases, REs close to 0% were observed, conveying a false sense of repeatability and reproducibility. Considering this, whole-body circulation model AIFs may provide greater accuracy than individual or pooled spleen AIFs when dealing with noisy signal data, however further investigation involving different organs, animals, and contrast agents is required. Moreover, fitting for the four whole-body circulation model parameters should ideally be performed to avoid misrepresentation of diseased states, where literature values do not accurately reflect the population of interest.CONCLUSION
The whole-body circulation model presented in this abstract is a robust alternative to using individual- or pooled-spleen data for deriving arterial input functions for DCE-MRI in small animals.Acknowledgements
This work is funded by Innovative Medicines Initiative 2 Joint Undertaking, Grant Agreement number 116106-IB4SD-TRISTAN. This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
References
1. Barnes SL, Whisenant JG, Loveless ME, Yankeelov TE. Practical dynamic contrast enhanced MRI in small animal models of cancer: data acquisition, data analysis, and interpretation. Pharmaceutics 2012;4(3):442-478.
2. Yang C, Karczmar GS, Medved M, Stadler WM. Estimating the arterial input function using two reference tissues in dynamic contrast‐enhanced MRI studies: fundamental concepts and simulations. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2004;52(5):1110-1117.
3. Ziemian S, Green C, Sourbron S, Jost G, Schutz G, Hines CDG. Ex vivo gadoxetate relaxivities in rat liver tissue and blood at five magnetic field strengths from 1.41 to 7 T. NMR in biomedicine 2021;34(1):e4401.
4. Scotcher D, Melillo N, Tadimalla S, Darwich AS, Ziemian S, Ogungbenro K, Schütz G, Sourbron S, Galetin A. Physiologically Based Pharmacokinetic Modeling of Transporter-Mediated Hepatic Disposition of Imaging Biomarker Gadoxetate in Rats. Molecular pharmaceutics 2021;18(8):2997-3009.
5. Cremer JE, Seville MP. Regional brain blood flow, blood volume, and haematocrit values in the adult rat. Journal of cerebral blood flow & metabolism 1983;3(2):254-256.
6. Lee HB, Blaufox MD. Blood volume in the rat. Journal of Nuclear Medicine 1985;26(1):72-76.
7. Jobin J, Bonjour J. Measurement of glomerular filtration rate in conscious unrestrained rats with inulin infused by implanted osmotic pumps. American Journal of Physiology-Renal Physiology 1985;248(5):F734-F738.
Figures
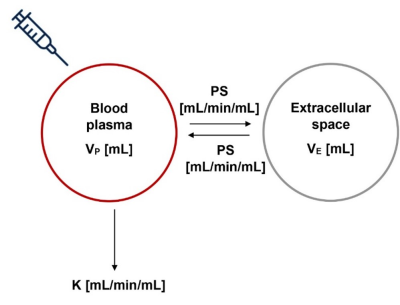
Tracer kinetic model for blood concentrations in rats. PS = whole body permeability-surface for Gadoxetate; VP = whole body plasma volume; VE = whole body extracellular volume; K = GFR + Ktrans*VL, where GFR is the glomerular filtration rate, Ktrans is the hepatic plasma clearance rate, and VL is the liver volume.