2758
Whole Brain Distortion-free 3D pseudo-Continuous Arterial Spin Labeling at 7T with Turbo FLASH, Optimized Labeling and Background Suppression1Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Siemens Medical Solutions USA, Los Angeles, CA, United States, 3Institute of Biomedical Imaging, Graz University of Technology, Graz, Austria, 4Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
Synopsis
Keywords: Arterial spin labelling, Arterial spin labelling, Perfusion
The potential of 7T has not been fulfilled for pseudo-Continuous Arterial Spin Labeling (pCASL) because of challenges in labeling, background suppression (BS), and readouts. This study proposed a new labeling parameter, a superior BS pulse, and an accelerated and segmented 3D Turbo-FLASH (TFL) readouts. Despite the field inhomogeneity at 7T, the new labeling parameter achieves high labeling efficiency without signal interference, and OPTIM as BS pulse robustly achieves high inversion efficiency. 3D TFL pCASL provides whole brain coverage, abundant anatomical information without distortion, high resolution, and sufficient SNR in 11 mins.Background
Ultrahigh-field offers potential advantages for pseudo-Continuous Arterial Spin Labeling (pCASL) with increased SNR and prolonged blood T11. However, this potential has not been fulfilled because of several challenges. Labeling efficiency (LE) is low due to the limited coverage of transmit coil. Increasing RF duty cycle and raising labeling position improve LE but may cause interference extending into imaging volume2. Background suppression (BS), which is crucial for segmented 3D readout, is limited by the low inversion efficiency (IE) due to field inhomogeneities. Workhorse readout sequences at 3T, such as GRASE and EPI, suffer from signal loss and distortion because of shortened T2/T2*. Here we present a whole brain distortion-free 3D pCASL sequence by combining high LE labeling, superior BS pulses, and accelerated Turbo-FLASH (TFL) readout at 7T.Methods
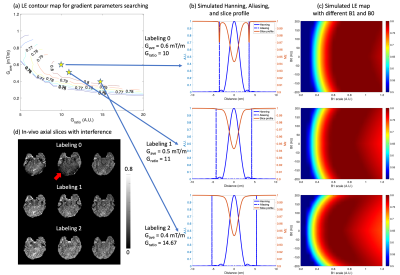
First, we dealt with sidelobe interferences of pCASL labeling in bottom imaging slices without compromising LE. To suppress sidelobe interferences, the ratio (Gratio) of max gradient, Gmax, to average gradient, Gave, must satisfy Eq.1 to overlap the first aliasing of the control pulse train with the kth zero-crossing of Hanning pulse as shown in Fig.1(b).$$G_{ratio} = \frac{2(k+1)T}{\tau} (k \in \mathbb Z^+)$$
Then, following the previous work2, the optimal gradient parameters were searched with the aforementioned constraint in a range of Gave=[0.05:0.05:1.2]mT/m and Gratio=[5:1:30] while fixing other parameters, including RF duration (τ)=300 us, duty cycle (T)=550 us, and flip angle (FA)=15°. Bloch simulation was performed with a halved B1 scaling according to the measured B1 around labeled arteries.
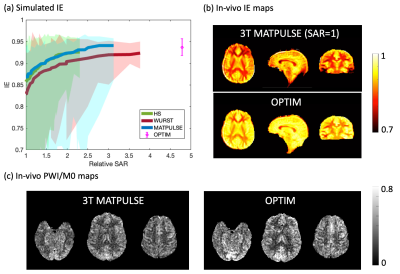
Second, we optimized BS pulses. Bloch simulation with various pulse-specific parameters was performed for three non-selective inversion BS pulses, including Hyperbolic Secant (HS)3, WURST4, and an optimal control-based pulse (MATPULSE)5. The OPTIM inversion pulse was designed as a free-form RF pulse using ensemble time optimal control techniques6,7. Mean, maximum, and minimum IE of each pulse achieved under B1 scaling=[0.35:1.15] and B0 offset=[-250:250]Hz were recorded in Fig.2(a).
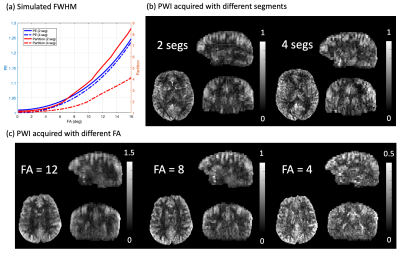
Third, we developed 3D TFL readout which was accelerated (R=2x2) following the CAIPIRINHA pattern and centric ordering. Various numbers of segments (2 and 4) and FAs (0-16°) were tested to demonstrate the trade-off between SNR and spatial blurring (FWHM). Simulation of point-spread function (PSF) and in-vivo experiments shared the same Imaging parameters.
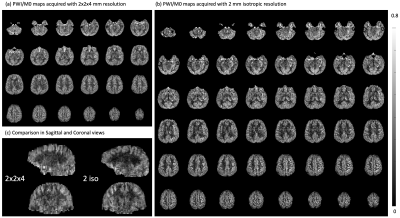
In-vivo experiments were performed for 7 healthy subjects (4 females, 25.6±3.2y/o) under IRB on the investigational pTx part of a Siemens 7T MAGNETOM Terra (Siemens Healthcare, Erlangen, Germany) with an investigational Nova 8Tx/32Rx head coil. Imaging parameters were: FOV=224×192×96mm3, matrix size=112×96×24, resolution=2×2×4mm3, TR=4ms, TE=1.55ms, 50 measurements acquired in 11min40sec. Evaluation of three Gave/Gratio pairs (6/10, 5/11, and 4/14.67) was performed on three subjects. OPTIM BS was evaluated on a subject and compared with MATPULSE that has been used at 3T and with relative SAR=1. Two subjects underwent experiments for TFL readout with FA=4/8/12° and 2/4 segments, respectively. To push towards higher resolution, 2mm3 isotropic pCASL images (48 slices, 4 segments, FA=12°, and 26 measurements in 12min10sec) were acquired on one subject and compared with the 2x2x4mm3 scan. Offline reconstruction was performed using an in-house GRAPPA program.
Results and discussion
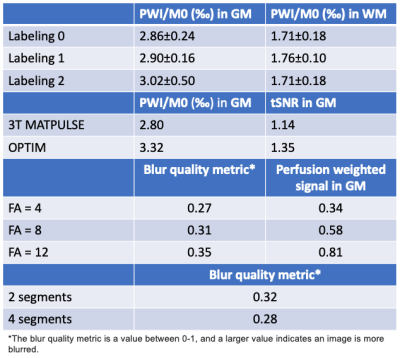
Figure 1(a) shows the simulated contour of LE and different gradient parameters. Two candidates, “labeling1” (0.5/11) and “labeling2” (0.4/14.67), achieved comparable LE as “labeling0” (0.6/10) and suppressed sidelobe interferences as shown in Fig.1(b). Fig.1(c) characterizes the two candidates with field inhomogeneities. With smaller Gave, the two candidates showed improved robustness to low B1 with a slightly increased sensitivity to B0 offset. “Labeling2” achieved the best suppression of interferences and 5% higher perfusion signal than “Labeling0” as indicated by Fig.1(d) and Table1.Figure 2(a) shows the simulated IE of four BS pulses. OPTIM achieved the best mean IE of 0.94 and min IE of 0.92 with a cost of 4.7 times higher SAR than the MATPULSE used at 3T8. Among the other 3 pulses, while MATPULSE had the best mean IE of 0.92 and 0.94 at relative SAR=2 and 3, respectively, all failed to maintain a min IE above 0.9. Figure 2(b) shows IE maps where 11% improvement can be achieved by OPTIM compared to 3T MATPULSE. With high IE, OPTIM improved the signal in PWI/M0 maps and tSNR displayed in Fig.2(c) and Table1 by 20%.
Figure 3(a) demonstrates the effect of FA and number of segments on FWHM. While no significant blurring was introduced in phase-encoding (PE) direction, perfusion weighted image (PWI) was blurred along partition/slice with an increased FA and a reduced number of segments. Blurring was measured in Table1 according to9, where the index increases as sharpness degrades. However, more segments aggravated sensitivity to motion and prolonged scan time, and perfusion signal decreased linearly with smaller FA. From Fig.3(b) and Fig.3(c), 2 segments and FA=8 were suggested.
Figure 4 (a) and (b) displays 2x2x4mm3 and iso-2mm3 PWI/M0 maps, respectively. Anatomical details throughout the whole brain, such as orbitofrontal cortex, choroid plexus, white matter, and cortical gyri, can be clearly seen. In Fig.4(c), finer through-plane details were revealed with higher resolution.
Conclusion
We presented a new set of labeling parameters, OPTIM BS pulse, and accelerated 3D TFL readout for pCASL at 7T. The technique provides whole brain coverage, detailed anatomical information without distortion, high resolution, and sufficient SNR in 11mins.Acknowledgements
This work is supported by National Institute of Health (NIH) grant R01-EB032169 and R01-EB028297.References
1. Zuo, Zhentao, et al. "Turbo-FLASH based arterial spin labeled perfusion MRI at 7 T." PloS one 8.6 (2013): e66612.
2. Wang, Kai, et al. "Optimization of pseudo‐continuous arterial spin labeling at 7T with parallel transmission B1 shimming." Magnetic resonance in medicine 87.1 (2022): 249-262.
3. Silver, Michael S., Richard I. Joseph, and David I. Hoult. "Highly selective π2 and π pulse generation." Journal of Magnetic Resonance (1969) 59.2 (1984): 347-351.
4. Kupce, E., and R. Freeman. "Adiabatic pulses for wideband inversion and broadband decoupling." Journal of Magnetic Resonance, Series A 115.2 (1995): 273-276.
5. Matson, G.B. An integrated program for amplitude-modulated RF pulse generation and re-mapping with shaped gradients. Magn. Reson. Imaging 12, 1205-1225, 1994.
6. Graf, Christina, et al. “Advanced design of MRI inversion pulses for inhomogeneous field conditions by optimal control”. NMR in Biomedicine 35(11), e4790, 2022.
7. Rund, Armin, et. Al, “Simultaneous multislice refocusing via time optimal control.” Magnetic Resonance in Medicine 80.4 (2018): 1416-1428.
8. Shao, Xingfeng, et al. "A constrained slice‐dependent background suppression scheme for simultaneous multislice pseudo‐continuous arterial spin labeling." Magnetic resonance in medicine 79.1 (2018): 394-400.
9. Crete, Frederique, et al. "The blur effect: perception and estimation with a new no-reference perceptual blur metric." Human vision and electronic imaging XII. Vol. 6492. SPIE, 2007.
Figures