2751
Dedicated ICTGV-regularized Reconstruction for time-encoded ASL1Institute of Biomedical Imaging, TU Graz, Graz, Austria, 2Department of Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands
Synopsis
Keywords: Arterial spin labelling, Brain
Time-encoded Arterial Spin Labeling (teASL) enables time-efficient acquisition of multiple averages of multiple post-labeling-delay (PLD) perfusion-weighted images (PWIs). We developed a dedicated teASL reconstruction that employs Infimal Convolution of Total Generalized Variation (ICTGV) regularization directly on the PWIs. Using simulated and healthy volunteer data, the ICTGV reconstruction produced inherently denoised images and improved cerebral blood flow and arterial transit time maps compared to a SENSE reconstruction. Also, for an 8-fold accelerated in-vivo dataset, the ICTGV reconstruction still produced reasonable PWIs, which the SENSE reconstruction could not. Therefore, ICTGV reconstruction is promising for teASL and allows high acceleration with inherent denoising.Introduction
Arterial Spin Labeling (ASL) is a non-invasive method for measuring brain perfusion with MRI and showed a strong development over the last few years. ASL continues to innovate$$$^1$$$ and is getting increasingly adopted into clinical practice.$$$^{2,3}$$$ One of the main obstacles ASL is still facing are long acquisition times due to the need for multiple averages, especially when acquiring multiple perfusion-weighted images (PWIs) with multiple post-labeling delays (PLDs). These are necessary to estimate arterial transit time (ATT), which increases the accuracy of cerebral blood flow (CBF) quantification.$$$^4$$$ Standard acceleration techniques like Parallel Imaging (PI) are commonly used in ASL, but only acceleration factors of 2-3 are recommended because of the intrinsically low Signal-to-Noise Ratio (SNR).$$$^2$$$ The introduction of time-encoded ASL (teASL) allows for time-efficient acquisition of several PWIs with different PLDs with a simultaneous averaging effect, further accelerating acquisition.$$$^{5,6}$$$ ASL-dedicated image reconstruction algorithms have shown great results by incorporating the ASL model or data structure directly in the reconstruction.$$$^{7,8}$$$. Infimal Convolution of Total Generalized Variation (ICTGV) regularization has been proven to work effectively in dynamic MR image reconstruction and allows for highly accelerated data acquisition with inherent denoising during reconstruction.$$$^{9}$$$ In this work, we propose a dedicated reconstruction for teASL which uses ICTGV regularization directly on the PWIs.Methods
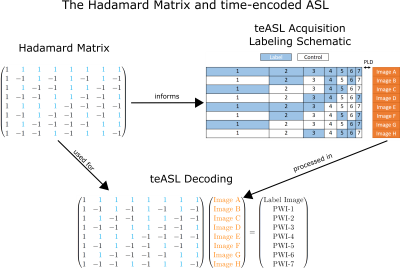
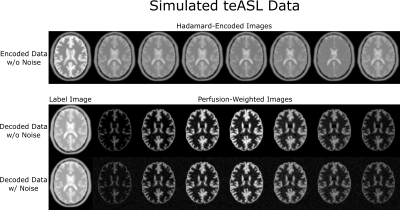
In teASL, $$$N$$$ images are labeled according to a Hadamard matrix during acquisition, which, after decoding, result in $$$N-1$$$ PWIs and an additional label image (Figure 1). In the proposed reconstruction, the PWIs are regularized with ICTGV, while the label image is regularized with Total Generalized Variation (TGV)$$$^{10}$$$,$$PWI^*=\text{argmin}_{PWI,label}\,\,\frac{\lambda}{2}||\mathcal{PFCH}(PWI,label)-d||_2^2+\theta_1\text{ICTGV}(PWI) +\theta_2\text{TGV}(label).$$This reconstruction problem is solved iteratively with a primal-dual algorithm.$$$^{11}$$$ The $$$\mathcal{PFCH}$$$ operator applies, in-order, the Hadamard encoding, coil sensitivities, Fourier transform and undersampling pattern. The $$$d$$$ variable stands for the k-space data, $$$\theta_{1,2}>0$$$ assign weighting between the TGV and ICTGV regularizations and $$$\lambda>0$$$ weighs data fidelity. To test the reconstruction, a teASL dataset using a 8x8 Hadamard matrix and two averages was simulated using the general kinetic model$$$^{4}$$$ and applied to a digital brain dataset$$$^{12}$$$ (Figure 2) and undersampled with a 2x2s1 CAIPIRINHA$$$^{13}$$$ pattern. Additionally, a teASL sequence with a single-shot 3D Gradient and Spin Echo (GRASE) readout capable of high CAIPIRINHA acceleration was developed and one healthy volunteer dataset was acquired on a Philips 3T Ingenia Elition X system. Acquisition parameters were as follows: TR/TE=3964/12 ms, EPI factor=15, TSE factor=16, Hadamard size=8, LD=3700ms divided into blocks of 1300/600/400/400/400/300/300ms, PLD=256ms, 12 averages, FOV: 240x240x102mm$$$^3$$$, voxel size=3.75x3.75x6mm$$$^3$$$, 4x2s1 CAIPIRINHA acceleration, 7:56min). The sampling pattern was shifted per scan repetition such that the first eight averages combine into a fully sampled k-space. Coil sensitivities were estimated using BART$$$^{14}$$$ and CBF and ATT maps were calculated using a non-linear-least-squares fit in Matlab.Results
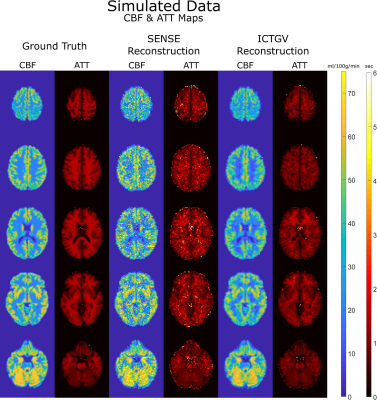
ICTGV reconstruction resulted in considerably improved image quality compared to the SENSE reconstruction for the 4-fold undersampled, simulated data (Figure 3). The ICTGV reconstruction yields much sharper and already denoised images. From the resulting PWIs, CBF and ATT maps were calculated (Figure 4). Again, the ICTGV maps are less noisy compared to the SENSE-derived images. The Root-Mean-Squared-Error (RMSE), compared to the ground truth, was 57% lower for CBF and 47% lower for ATT with ICTGV compared to SENSE reconstruction. A SENSE and ICTGV reconstruction of the prospectively 4x2s1 undersampled single-shot 3D GRASE teASL scan in a healthy volunteer is shown in Figure 5.Discussion
In this work, we have shown the feasibility of ICTGV reconstruction for teASL. The advantages of the proposed method are twofold. First, the inherent denoising capabilities of ICTGV improve the quality of both the PWIs and subsequent CBF and ATT maps without the need for additional denoising in post-processing. Second, although our in-vivo dataset still suffered from serious phase errors caused by the EPI readout, the images still show the other advantage of our approach: ICTGV reconstruction can reconstruct heavily accelerated teASL data. While the SENSE reconstruction could not produce usable images from the 8-fold accelerated, single-shot dataset, the ICTGV reconstruction produced PWIs with reasonable quality. This potentially enables acceleration factors of more than twice the currently recommended$$$^2$$$ acceleration for teASL, shortening the data acquisition or producing higher resolution images in the same scan time. Surprisingly, using different undersampling patterns per 3D image within a Hadamard series resulted in artifacts interfering with the reconstruction. Therefore, the sampling pattern was varied per repetition and images were reordered in image-space to create the same effect during reconstruction as altering the undersampling pattern for each frame.Conclusion & Outlook
In this work, we show the potential of ICTGV reconstruction for teASL. This novel method allows for higher acceleration factors than SENSE reconstruction, while already including denoising within the reconstruction. With these promising results, we aim to resolve the phase errors in the prospectively undersampled 3D GRASE acquisition and include rigid motion correction (MoCo) into the reconstruction. This would further enhance the quality of in-vivo data as motion errors could no longer interfere with the temporal regularization. Also, as MoCo is a common feature in ASL post-processing pipelines, this would simplify the teASL imaging processing.Acknowledgements
No acknowledgement found.References
[1] Qin, Qin, et al. "Velocity‐selective arterial spin labeling perfusion MRI: A review of the state of the art and recommendations for clinical implementation." Magnetic resonance in medicine 88.4 (2022): 1528-1547.
[2] Alsop, David C., et al. "Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia." Magnetic resonance in medicine 73.1 (2015): 102-116.
[3] Ho, Mai-Lan. "Arterial spin labeling: clinical applications." Journal of Neuroradiology 45.5 (2018): 276-289.
[4] Buxton, Richard B., et al. "A general kinetic model for quantitative perfusion imaging with arterial spin labeling." Magnetic resonance in medicine 40.3 (1998): 383-396.
[5] Günther, M. "Highly efficient accelerated acquisition of perfusion inflow series by cycled arterial spin labeling." Proc Intl Soc Mag Reson Med. Vol. 15. 2007. (first teASL)
[6] Wells, Jack A., et al. "In vivo Hadamard encoded continuous arterial spin labeling (H‐CASL)." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 63.4 (2010): 1111-1118.
[7] Spann, Stefan M., et al. "Robust single-shot acquisition of high resolution whole brain ASL images by combining time-dependent 2D CAPIRINHA sampling with spatio-temporal TGV reconstruction." NeuroImage 206 (2020): 116337.
[8] Maier, Oliver, et al. "Non-linear fitting with joint spatial regularization in arterial spin labeling." Medical Image Analysis 71 (2021): 102067.
[9] Schloegl, Matthias, et al. "Infimal convolution of total generalized variation functionals for dynamic MRI." Magnetic resonance in medicine 78.1 (2017): 142-155.
[10] Knoll, Florian, et al. "Second order total generalized variation (TGV) for MRI." Magnetic resonance in medicine 65.2 (2011): 480-491.
[11] Chambolle, Antonin, and Thomas Pock. "A first-order primal-dual algorithm for convex problems with applications to imaging." Journal of mathematical imaging and vision 40.1 (2011): 120-145.
[12] Liu, F. et al. Fast Realistic MRI Simulations Based on Generalized Multi-Pool Exchange Tissue Model. IEEE Transactions on Medical Imaging. 2016.doi: 10.1109/TMI.2016.2620961
[13] Breuer, Felix A., et al. "Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA)." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 55.3 (2006): 549-556.
[14] BART Toolbox for Computational Magnetic Resonance Imaging, DOI: 10.5281/zenodo.592960
Figures